��Ŀ����
п�������ǻ��ý��������������ﶼ��������ǿ����������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������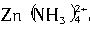 ��
��
����д���пհף�
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ_____________���û�ѧʽ��ʾ����
��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ��______________________��
��3�����и����е��������ʵ���Һ������μӵ�ʵ�鷽�����ɼ������______��
������������������ ���������Ͱ�ˮ ������п���������� ������п�Ͱ�ˮ
��4��д�������������백ˮ��Ӧ�����ӷ���ʽ��_____________________���Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��_______
________________________________��

��2��Zn+2NaOH=Na2ZnO2+H2���ۻ�Zn+2NaOH+2H2O=Na2Zn��OH��4+H2����
��3���٢ۢ�
��4��Al3++3NH3?H2O=Al��OH��3��+3NH4+��������п���백ˮ��Ӧ������Zn��OH��2�����ڹ�����ˮ�У�����Zn��NH3��42+���Ұ�ˮ������������
�����������
������������һ���������ϢǨ���⡣���������Ǿ������Ե��������ͨ������֪ʶ��Ǩ�ƣ�ͬѧ�ǿ��Ը��õ�����������п�����ԡ�
��1����������������������Һ������ƫ�����ƣ�����Һ����Ԫ�صĴ�����ʽΪ
 ��
����2�����շ�Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2��������д��Zn������������Һ��Ӧ�Ļ�ѧ����ʽ��Zn+2NaOH=Na2ZnO2+H2������Ǩ����Ҫע������п��̬�IJ�ͬ��
��3������μӵ�ʵ�鷽�������Լ���Ҫ�����Լ������Ӧ����ǰ�ߵ�����ߺͺ��ߵ���ǰ�ߵķ�Ӧ����Ӧ��ͬ����������Ϣ��֪��Al��OH��3��Zn��OH��2��Ϊ������������������ᷴӦ������Ӧ������ͬ���ǣ�Al��OH��3���ܱ���ˮ�ܽ⣬Zn��OH��2�ܱ���ˮ�ܽ⡣
Al2��SO4��3��Һ����NaOH��Һ�У�������AlO2-�������ɳ�����NaOH��Һ����Al2��SO4��3��Һ���г������ɺ����ܽ⣻Al2��SO4��3��Һ���백ˮ���г������ɣ���ˮ����Al2��SO4��3��Һ��Ҳ�г������ɣ�ZnSO4��Һ����NaOH��Һ��û�г������ɣ�����
 ��NaOH��Һ����ZnSO4��Һ�����г������ɺ����ܽ⣻ZnSO4��Һ���백ˮ��û�г������ɣ�����
��NaOH��Һ����ZnSO4��Һ�����г������ɺ����ܽ⣻ZnSO4��Һ���백ˮ��û�г������ɣ����� ����ˮ����ZnSO4��Һ�����г������ɺ����ܽ⡣
����ˮ����ZnSO4��Һ�����г������ɺ����ܽ⡣��4��������֪���������������ڰ�ˮ����ˣ������������백ˮ��Ӧ�����ӷ���ʽ����д������������п�����ڰ�ˮ��
 ���ɴ˿ɽ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��
���ɴ˿ɽ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ�� 
��ϰ��ϵ�д�
�����Ŀ
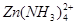 ��
�� ���ش��������⣺
���ش��������⣺