��Ŀ����
(10�֣�п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������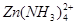 ��
��
�ش��������⣺
��1��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ
___________________________________________________ ��
��2�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������ ��
������������������ ���������Ͱ�ˮ
������п���������� ������п�Ͱ�ˮ
��3��д�������������백ˮ��Ӧ�����ӷ���ʽ
______________________________________________________ ��
��4���Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��
__________________________________________ ________________��
��1��Zn+2NaOH===Na2ZnO2+H2�� ��2�֣�
��2���٢ۢ� ��2�֣�
��3��Al3++3NH3��H2O=== Al(OH)3 ��+3NH4+ ��2�֣�
��4��������п���백ˮ��Ӧ������Zn(OH)2 �����ڹ�����ˮ�У�����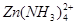 ����ˮ�����������ơ���4�֣�
����ˮ�����������ơ���4�֣�
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ��
�� ��
��