��Ŀ����
19������������ɼ��ʱ��������������ѧ����Ϊ������ȼ�ϵ��ŷ������¹��ش���ȶ��Ļ����ﲻ�Ǽ��顢һ���������������̼������ˮ��ˮ������Ӱ�������������壮�����й�˵������ȷ���ǣ�������| A�� | ���顢������̼��ˮ�����ɼ��Լ����ɵķǼ��Է��� | |
| B�� | һ���������Ͷ�����̼Ϊ�ȵ����壬���ǵ�Ħ����������44g | |
| C�� | �ڶ�����̼�����У���ÿ��������̼���Ӿ������������Ķ�����̼������6�� | |
| D�� | �ڱ��У�ÿ��ˮ���������ڵ��ĸ�ˮ�����γ�4����� |
���� A��ˮ�Ǽ��Է��ӣ�
B��ԭ�����ͼ۵�������ͬ����Ϊ�ȵ����壬Ħ�������ĵ�λΪg/mol��
C��������X��Y��Z�����и�ķ����ж϶�����̼���ӵĸ�����
D��ˮ������һ��O���������ӵ�Hԭ���γ�2�������2��Hԭ���������ˮ�����е�Oԭ���γ������
��� �⣺A�����顢������̼���ɼ��Լ����ɵķǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ���A����
B��ԭ�����ͼ۵�������ͬ����Ϊ�ȵ����壬һ���������Ͷ�����̼Ϊ�ȵ����壬���ǵ�Ħ����������44g/mol����B����
C��������X��Y��Z�����и�ķ����ж϶�����̼���ӵĸ���Ϊ12��������CO2�����У���ÿ��CO2������Χ���ڵ���12��CO2���ӣ���C����
D��ˮ�����к���һ��Oԭ�Ӻ�2��Hԭ�ӣ�ˮ������һ��O���������ӵ�Hԭ���γ�2�������2��Hԭ���������ˮ�����е�Oԭ���γ����������ÿ��ˮ���������ڵ��ĸ�ˮ�����γ�4���������D��ȷ��
��ѡD��
���� ���⿼���˷��ӵļ��ԡ��ȵ����塢Ħ������������������ȣ���Ŀ�漰��֪ʶ��϶࣬�����ڻ���֪ʶ�Ŀ��飬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
7����ͼΪԪ�����ڱ���һ���֣�W��X��Y��Z��Ϊ����������Ԫ�أ����з���һ����ȷ���ǣ�������
| W | X | |
| Y | Z |
| A�� | Y����̬�⻯�����ȶ� | B�� | Z�ĵ��������ӻ�ԭ����ǿ | ||
| C�� | X���ʳ����»�ѧ���ʻ��� | D�� | Y��ԭ��������W��7 |
4��������25��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ�������
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ksp��Ka | Ksp=1.8��10-10 | Ksp=9.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ����Һ�У���������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ����������Һ��ͨ������CO2�����ӷ���ʽΪ��2ClO-+CO2+H2O=CO32-+2HClO | |
| C�� | ��0.1 mol•L-1CH3COOH��Һ�еμ�NaOH��Һ����c��CH3COOH����c��CH3COO-��=5��9����ʱ��Һ��pH=5 | |
| D�� | ��Ũ�Ⱦ�Ϊ1.0��10-3 mol•L-1��KCl��K2CrO4�����Һ�еμ�1.0��10-3 mol•L-1��AgNO3��Һ��CrO42-���γɳ��� |
8�����������к��зǼ��Լ��Ĺ��ۻ������ǣ�������
| A�� | CCl4 | B�� | Na2O2 | C�� | C2H4 | D�� | CS2 |
9��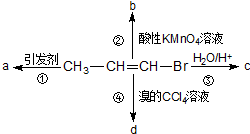 1-���ϩ�ܷ�������ͼ��ʾ��4����ͬ��Ӧ����֪����aΪ�߷��ӻ�����������ֻ����һ�ֹ����ŵķ�Ӧ�ǣ�������
1-���ϩ�ܷ�������ͼ��ʾ��4����ͬ��Ӧ����֪����aΪ�߷��ӻ�����������ֻ����һ�ֹ����ŵķ�Ӧ�ǣ�������
 1-���ϩ�ܷ�������ͼ��ʾ��4����ͬ��Ӧ����֪����aΪ�߷��ӻ�����������ֻ����һ�ֹ����ŵķ�Ӧ�ǣ�������
1-���ϩ�ܷ�������ͼ��ʾ��4����ͬ��Ӧ����֪����aΪ�߷��ӻ�����������ֻ����һ�ֹ����ŵķ�Ӧ�ǣ�������| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢� |
 ��
�� ��������������һϵ�����ʣ��йر仯��ͼ��
��������������һϵ�����ʣ��йر仯��ͼ��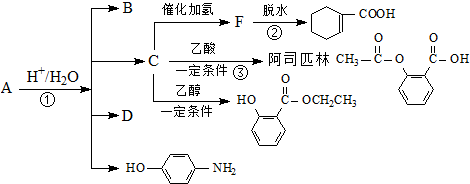
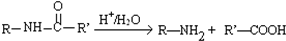
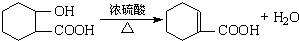 ��
�� ��
��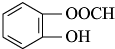 ����������
�����λ���λ����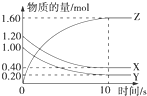 ��1��ij�¶��£�2L�����ܱ������У�X��Y��Z�������巢����ѧ��Ӧʱ�����ʵ�����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����
��1��ij�¶��£�2L�����ܱ������У�X��Y��Z�������巢����ѧ��Ӧʱ�����ʵ�����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����