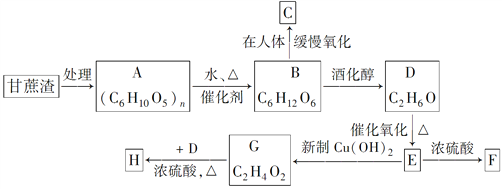
��Ŀ����
����Ŀ��ij���dz��Ը���Ϊԭ�����ǣ�ͬʱ�õ������ĸ��������Ը�������ۺ����ã�����������߾���Ч�棬���һ��ܷ�ֹ������Ⱦ���ְ����·�ʽ���У�
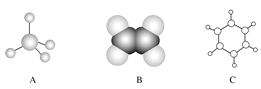
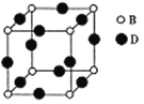
��֪F��H���Ǿ�����ζ��Һ�壬FΪE�����ۺ�������������Ԫ��״�Գƽṹ������գ�
��1��A������________��D��ͬ���칹��Ľṹ��ʽΪ________________��
��2��E��G�Ļ�ѧ����ʽ_________________________��
��3��G��H�Ļ�ѧ����ʽ_________________________��
��4��F�Ľṹ��ʽ��_____________________��
���𰸡� ��ά�� CH3��O��CH3 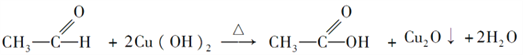 CH3CH2OH+CH3COOH
CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O 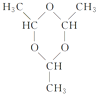
��������������A�ķ���ʽ��C6H10O5��n��֪A���ǵ��۾�����ά�أ���ϵ����ʵ��ȷ��AΪ��ά�أ�BΪ�����ǣ�DΪ�ƾ���EΪ��ȩ��FΪE��������Ҿ����������Ԫ��״�Գƽṹ���������ݳɼ�ԭ��ֻ����C=O��ʽ�ӳ����ɡ���ͼ��ʾ��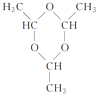 ����1��A��������ά�أ�DΪ�ƾ�����ͬ���칹��Ľṹ��ʽΪCH3��O��CH3����2��E��G����ȩ�����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��
����1��A��������ά�أ�DΪ�ƾ�����ͬ���칹��Ľṹ��ʽΪCH3��O��CH3����2��E��G����ȩ�����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��
��3��G��H������Ҵ���Ũ����Ĵ��·�Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O����4��F�Ľṹ��ʽΪ��
CH3COOCH2CH3+H2O����4��F�Ľṹ��ʽΪ�� ��
��

����Ŀ����������������Ҫ�Ŀ�����Ⱦ����Ŀǰ��������������Ⱦ�ķ����ж��֡�
(1)��CH4����ԭ��������������������������Ⱦ��
CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160 kJ��mol��1������
CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574 kJ��mol��1������
H2O(g)��H2O(l) ��H����44��0 kJ��mol��1������
��д��CH4 (g)��NO2 (g)��Ӧ����N2 (g) ,CO2(g)��H2O(l)���Ȼ�ѧ����ʽ��________
��Ϊ�о���ͬ�����¶�������Ӧ( �� )��Ӱ�죬�ں���������,��2 L �ĺ����ܱ������м���0.2mol CH4��0.4mol NO2,10min��Ӧ�������ﵽƽ�⣬���l0min��v(NO)=5��10-3mol/(L��min),��ƽ���n(CH4)=___mol,NO2��ת����a1=_________.�����������䣬��Ӧ�ں�ѹ�����½��У�ƽ��ʱNO2��ת����a2____a1(����ڡ� С�ڡ��� ���ڡ� )��
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g)![]() N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
ʱ�� Ũ��(mol/L) ���� | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
�ٲ�����Ϊ�жϷ�Ӧ�ﵽ��ѧƽ��״̬������ ��_________
A��������CO2��Ũ�ȱ��ֲ���
B��v����N2��= v����NO��
C����������ƽ����Է����������ֲ���
D�����������ܶȱ��ֲ���
E��������ѹǿ���ֲ���
����T��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ_______ (������λС��)��
����30 min,�ı�ijһ����,��Ӧ���´ﵽƽ��,��ı��������________��
��3����ѧ�������о����ô������������ٷɻ�β���е�NO��COת���CO2��N2, �о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ���ͼ��ʾ��������������ʱ����Ӧ��2CO(g)��2NO(g)![]() N2(g)��2CO2(g) ��NO��Ũ��c(NO)���¶�(T)�����������(S)��ʱ��(t)�ı仯���ߡ�
N2(g)��2CO2(g) ��NO��Ũ��c(NO)���¶�(T)�����������(S)��ʱ��(t)�ı仯���ߡ�

�ٸ÷�Ӧ�Ħ�H____0���>����<������
���������ı����S1 >S2����ͼ�л���c(NO)��T1��S2�����´ﵽƽ������еı仯���ߡ�____