��Ŀ����
A��B��C��D��EΪԭ������������������ֶ�����Ԫ�أ�A��Eͬ���壬A����B��C��D�ֱ��γɵ�������ȵ����ַ��ӣ�C��D����������֮����E�ĺ������������ȣ�
�Իش�
��1��������ABC�ЦҼ��ͦм�������Ϊ
 ��
��
��2��A��D��E ����Ԫ�ؿ��γ�һ�����ӻ�����R���ö��Ե缫��� R ��ϡ��Һ�����һ��ʱ�����Һ pH
��3����0.1mol?L-1E2BD3����Һ�У�������Ũ���ɴ�С��˳����
��4��ij�ܱ������з������·�Ӧ��C2��g��+3A2��g��?2CA3��g������H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����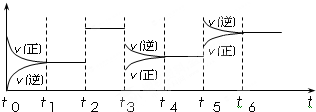
A��t5ʱ������ѹǿ B��t3ʱ�������¶�
C��t2ʱ�����˴��� D��t4��t5ʱ����ת����һ�����
��5������A��ȼ����ΪakJ/mol��12gB����ȼ�����ɻ�����BD�ų�����bkJ��1mol������A2D��Һ̬��Ϊ��̬����������ckJ��д������B��ˮú�����Ȼ�ѧ����ʽ
�Իش�
��1��������ABC�ЦҼ��ͦм�������Ϊ
1��1
1��1
������������
����
���ӣ�����ԡ��Ǽ��ԡ�����������C2A2��һ�ָ��������ӣ���C ԭ������8 �����ȶ��ṹ��д��C2A2�ĵ���ʽ

��2��A��D��E ����Ԫ�ؿ��γ�һ�����ӻ�����R���ö��Ե缫��� R ��ϡ��Һ�����һ��ʱ�����Һ pH
����
����
�����������С�����䡱������3����0.1mol?L-1E2BD3����Һ�У�������Ũ���ɴ�С��˳����
c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��
c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��
������ʽΪ A2B2D4�Ļ����������ʵ����� KOH ��Һ��Ӧ����Һ�����ԣ���ԭ���ǣ���������գ�HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶�
HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶�
����4��ij�ܱ������з������·�Ӧ��C2��g��+3A2��g��?2CA3��g������H��0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����
C
C

A��t5ʱ������ѹǿ B��t3ʱ�������¶�
C��t2ʱ�����˴��� D��t4��t5ʱ����ת����һ�����
��5������A��ȼ����ΪakJ/mol��12gB����ȼ�����ɻ�����BD�ų�����bkJ��1mol������A2D��Һ̬��Ϊ��̬����������ckJ��д������B��ˮú�����Ȼ�ѧ����ʽ
C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol
C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol
��������A����B��C��D�ֱ��γɵ�������ȵ����ַ��ӣ�B��C��DΪ��Ϊ�⻯�����10�����ӻ�18�����ӣ���AӦΪHԪ�أ�A��Eͬ���壬��E��ԭ���������ӦΪNaԪ�أ�C��D����������֮����E�ĺ������������ȣ��ܹ�Ϊ11����CΪNԪ�أ�DΪOԪ�أ���ԭ����������������B���γ��⻯���֪BΪCԪ�أ����Ԫ�ض�Ӧ�ĵ��ʣ�����������ʽ����⣮
����⣺A����B��C��D�ֱ��γɵ�������ȵ����ַ��ӣ�B��C��DΪ��Ϊ�⻯�����10�����ӻ�18�����ӣ���AӦΪHԪ�أ�A��Eͬ���壬��E��ԭ���������ӦΪNaԪ�أ�C��D����������֮����E�ĺ������������ȣ��ܹ�Ϊ11����CΪNԪ�أ�DΪOԪ�أ���ԭ����������������B���γ��⻯���֪BΪCԪ�أ�
��1��������ABCΪHCN���ṹʽΪHC��N������2���Ҽ���2���м������߱�ֵΪ1��1��Ϊ���Է��ӣ�������C2A2ΪN2H2��
Nԭ������8�����ȶ��ṹ������ʽӦΪ ���ʴ�Ϊ��1��1�����ԣ�
���ʴ�Ϊ��1��1�����ԣ� ��
��
��2��A��D��E ����Ԫ�ؿ��γ�һ�����ӻ�����RΪNaOH�����ʱ��ҺŨ������pH���ʴ�Ϊ������
��3��E2BD3ΪNa2CO3��Ϊǿ�������Σ�ˮ��ʼ��ԣ��Ե�һ��ˮ��Ϊ������Һ������Ũ�ȴ�С˳��Ϊ
c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��������ʽΪH2C2O4�Ļ����������ʵ����� KOH ��Һ��Ӧ����KHC2O4����Һ�д���HC2O4-�ĵ����ˮ�⣬��Ӧ����Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶ȣ�
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶ȣ�
��4����ͼ���֪��
A��t5ʱ�淴Ӧ���ʴ�������Ӧ���ʣ��ҷ�Ӧ���ʶ�����ӦΪ�����¶ȣ���������ѹǿ������Ӧ����Ӧ�����淴Ӧ���ʣ���A����
B��t3ʱ���淴Ӧ���ʶ���С�����淴Ӧ���ʴ�������Ӧ���ʣ�Ӧ�ǽ���ѹǿ��ԭ��B����
C��t2ʱ���淴Ӧ���ʶ�������ƽ�ⲻ�ƶ���Ӧ�Ǽ����˴�������C��ȷ��
D��t5��t6ʱ����ת����һ����ͣ���D����
�ʴ�Ϊ��C��
��5����֪����H2��ȼ����ΪakJ/mol��12gC����ȼ�����ɻ�����CO�ų�����bkJ��1mol������H2O��Һ̬��Ϊ��̬����������ckJ��
��ɵ��Ȼ�ѧ����ʽ�ֱ�Ϊ����H2��g��+
O2��g��=H2O��l����H=-akJ/mol��
��C��s��+
O2��g��=CO��g����H=-bkJ/mol��
��H2O��l��=H2O��g����H=+ckJ/mol��
�����ø�˹���ɽ���-��-�ۿɵ�C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol��
�ʴ�Ϊ��C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol��
��1��������ABCΪHCN���ṹʽΪHC��N������2���Ҽ���2���м������߱�ֵΪ1��1��Ϊ���Է��ӣ�������C2A2ΪN2H2��
Nԭ������8�����ȶ��ṹ������ʽӦΪ
 ���ʴ�Ϊ��1��1�����ԣ�
���ʴ�Ϊ��1��1�����ԣ� ��
����2��A��D��E ����Ԫ�ؿ��γ�һ�����ӻ�����RΪNaOH�����ʱ��ҺŨ������pH���ʴ�Ϊ������
��3��E2BD3ΪNa2CO3��Ϊǿ�������Σ�ˮ��ʼ��ԣ��Ե�һ��ˮ��Ϊ������Һ������Ũ�ȴ�С˳��Ϊ
c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��������ʽΪH2C2O4�Ļ����������ʵ����� KOH ��Һ��Ӧ����KHC2O4����Һ�д���HC2O4-�ĵ����ˮ�⣬��Ӧ����Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶ȣ�
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����HC2O4-�ĵ���̶ȴ���HC2O4-��ˮ��̶ȣ�
��4����ͼ���֪��
A��t5ʱ�淴Ӧ���ʴ�������Ӧ���ʣ��ҷ�Ӧ���ʶ�����ӦΪ�����¶ȣ���������ѹǿ������Ӧ����Ӧ�����淴Ӧ���ʣ���A����
B��t3ʱ���淴Ӧ���ʶ���С�����淴Ӧ���ʴ�������Ӧ���ʣ�Ӧ�ǽ���ѹǿ��ԭ��B����
C��t2ʱ���淴Ӧ���ʶ�������ƽ�ⲻ�ƶ���Ӧ�Ǽ����˴�������C��ȷ��
D��t5��t6ʱ����ת����һ����ͣ���D����
�ʴ�Ϊ��C��
��5����֪����H2��ȼ����ΪakJ/mol��12gC����ȼ�����ɻ�����CO�ų�����bkJ��1mol������H2O��Һ̬��Ϊ��̬����������ckJ��
��ɵ��Ȼ�ѧ����ʽ�ֱ�Ϊ����H2��g��+
| 1 |
| 2 |
��C��s��+
| 1 |
| 2 |
��H2O��l��=H2O��g����H=+ckJ/mol��
�����ø�˹���ɽ���-��-�ۿɵ�C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol��
�ʴ�Ϊ��C��s��+H2O��g��=CO��g��+H2��g����H=��a-b-c��kJ/mol��
���������⿼��ԭ�ӽṹ��Ԫ��������֪ʶ����Ŀ�Ѷ��еȣ������ʱע�����ԭ�Ӻ�������Ų���ϵ��ѧϰ��ע�����ø�˹���ɼ��㷴Ӧ�ȵķ�����

��ϰ��ϵ�д�
�����Ŀ
����ѧ--ѡ��3�����ʽṹ�����ʡ�
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��1��Gλ�� �� �����۵����Ų�ʽΪ ��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ ��
��4����֪BA5Ϊ���ӻ����д�������ʽ ��
��5��DE3����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ ��
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ��� ��
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�

