��Ŀ����
��6�֣���a��b����������Ҽ����ȵ��ܱ�������a�����ݻ����䣬b�еĻ����������ƶ����Ա�������ѹǿ��ȡ�����ͬ�����½�3mol
A��1mol B�ֱ�ͬʱ�����a��b�������У�������Ӧ��3A(g)��B(g)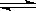 2C(g)��D(g)
2C(g)��D(g)
��1���ﵽƽ��ʱ��a��A��Ũ��ΪM mol/L��A��ת����ΪN��b��A��Ũ��Ϊm mol/L��A��ת����Ϊn����M____m��N_____n����������������Ƚϣ�
��2�������¶Ȳ��䣬��������ȷֱ����a��b����������ƽ���a��A��Ũ��ΪM mol��L��1����
A 6mol A��2mol B B 3mol A��2mol C C 2mol C��1mol B��1mol D
D 2mol C��1mol D E 1.5mol C��0.5mol B��1mol C��0.5mol D
��1���� �� ��2��D��E
����������1����Ӧ��һ�������С�Ŀ��淴Ӧ���ڷ�Ӧ������b�е�ѹǿʼ�մ���a�е�ѹǿ������b�е�ת���ʴ���a�еġ�����b�����С������A��Ũ�ȴ���a�еġ�
��2��a�DZ����������ģ�����Ҫʹƽ���Ч�����������ʵ����ʵ������������ͬ��ѡ��C�൱��3mol A��2mol B��ѡ��D�൱����3mol A��1mol B��ѡ��E�൱����3mol A��1mol B�����Դ���DE��


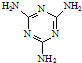 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 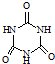 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ�� 2C(g)��D(g)
2C(g)��D(g) 2C(g)��D(g)
2C(g)��D(g)