��Ŀ����
2013���������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g)+2CO
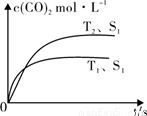
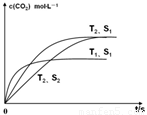
(g) 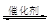 2CO2 (g) +N2 (g) ���ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ���ͼ��ʾ���ݴ��жϣ�
2CO2 (g) +N2 (g) ���ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ���ͼ��ʾ���ݴ��жϣ�
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ ��
�ڸ÷�Ӧ�Ħ�H 0��ѡ�����������������
�۵��������������һ��ʱ����������������� ѧ��Ӧ���ʡ��������ı����S1��S2����ͼ�л���c(CO2)��T2��S2�����´ﵽƽ������еı仯���ߡ�
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
�� úȼ�ղ����������������������CH4����ԭNOx�������������������
Ⱦ��
CH4(g)��2NO2(g)
= N2(g)��CO2(g)��2H2O(g) 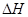 =��867kJ��mol��1
=��867kJ��mol��1
2NO2(g) 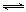 N2O4(g)
N2O4(g)
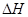 =��56.9kJ��mol��1
=��56.9kJ��mol��1
д��CH4����ԭN2O4(g)����N2(g)��CO2(g)��H2O(g)���Ȼ�ѧ����ʽ ��
�� ��ȼú�����Ķ�����̼�������ã��� �ﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ�����ת��ԭ��������ԭ���Ʊ��²�Ʒ��ʾ��ͼ��д���������ת�����̵Ļ�ѧ��Ӧ����ʽ ������a��b֮�����ӵ����ϵ������������� (��a��b��b��a) ��
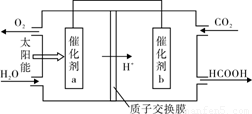
��1����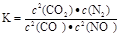 ��2�֣� �ڣ���1�֣�
��2�֣� �ڣ���1�֣�
�ۣ�2�֣�[�����ԭ��0������1�֣����ﵽƽ���ʱ����ڡ�T2��S1�����ߵ�ƽ��㣨��1�֣�
��2���� CH4(g)��N2O4(g) = N2(g)��CO2(g)��2H2O(g)
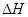 =��810.1 kJ��mol��1��2�֣�
=��810.1 kJ��mol��1��2�֣�
��2CO2��2H2O 2HCOOH+O2��2�֣� a��b ��2�֣�
2HCOOH+O2��2�֣� a��b ��2�֣�
��������
�����������1���ڷ�Ӧͼ��ó�TI���߷�Ӧ���ʿ죬�¶ȸߣ��������̼�����ͣ���Ӧ���ȣ��۱����Խ��Ӧ����Խ�죬���������ı�ƽ��Ũ�ȣ�ƽ��ʱ������̼Ũ����ͬ����2���ٷ���ʽ1������ʽ2��CH4(g)��N2O4(g)
= N2(g)��CO2(g)��2H2O(g) 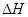 =��810.1 kJ��mol��1���ڴӵ��ʾ��ͼ�ó���Ӧ��Ϊˮ�Ͷ�����̼��������Ϊ�����������ˮ�ڴ���aʧȥ��������������aΪ������������������a��b��
=��810.1 kJ��mol��1���ڴӵ��ʾ��ͼ�ó���Ӧ��Ϊˮ�Ͷ�����̼��������Ϊ�����������ˮ�ڴ���aʧȥ��������������aΪ������������������a��b��
���㣺���黯ѧ��Ӧԭ���й����⡣











