��Ŀ����
������ԭ�ζ�ʵ��ͬ�к͵ζ�����(����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮)������0.001 mol��L��1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2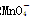 ��
��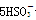 ��H��===2Mn2����5
��H��===2Mn2����5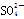 ��3H2O����ջش����⣺
��3H2O����ջش����⣺
(1)�õζ�ʵ�����������������е�________(�����)��
| A����ʽ�ζ���(50 mL) | B����ʽ�ζ���(50 mL) |
| C����Ͳ(10 mL) | D����ƿ |
G���ձ� H����ֽ
I����ͷ�ι� J��©��
(2)����________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��
________________________________________________________________________��
(3)ѡ����ָʾ����˵������
________________________________________________________________________��
(4)�ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����(b��a)mL��ʵ������KMnO4��Һ���ƫ________(��ࡱ���١�)������(b��a) mL����õ��Ĵ���Ũ�ȣ���ʵ��Ũ��ƫ________(���С��)��
(1)ABDEFH
(2)����������ǿ�������ܸ�ʴ��
(3)����ָʾ������Ϊ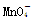 Mn2��ʱ��ɫ��ȥ
Mn2��ʱ��ɫ��ȥ
(4)�١�С
�������������(1)��Ϊ������ԭ�ζ�ʵ���������к͵ζ������к͵ζ�ʵ������������ѡ�ý���Ǩ�ƿɵó���ȷ�𰸡�
(2)����KMnO4����ǿ�����ԣ��ܸ�ʴ�ܣ��ʲ����ü�ʽ�ζ���ʢ��KMnO4��Һ��
(3)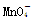 Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣
Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣
(4)�ζ�����Һ�棬�������ƫС������Ũ�ȱ�ʵ��Ũ��ƫС��
���㣺����ζ�ʵ����жϡ������Լ��йص�������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�ʵ����
��1���ռ��ڱ�����̻Ჿ�ֱ��ʣ�������ҪΪNa2CO3����
ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ����ȡ���ƺõ��ռ���Һ10.00mL����ƿ�У��ֱ�����ƿ�и�����1��2�η�ָ̪ʾ��������֪����̪��ɫʱ����ʱֻ��NaOH��HCl��Ӧ��Na2CO3��û����HCl��Ӧ����Ũ��Ϊ0.20mol��L-1�������Һ���еζ���������ݼ�¼���£�
|
ʵ���� |
V���ռ���Һ��/mL |
V�����ᣩ/mL |
|
|
��ʼ���� |
ĩβ���� |
||
|
1 |
10.00 |
0.50 |
21.52 |
|
2 |
10.00 |
1.00 |
21.98 |
|
3 |
10.00 |
0.20 |
24.80 |
�Իش�
�ٵζ�ʱ���ֵ���ȷ������______________________________________��
���жϵ���ζ��յ��ʵ��������__________________________________________�����ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ______ _����
�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���__________��
A����ƿ������ˮϴ��δ�ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ζ�����������ƿʱ��������������Һ����
D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
��2��������ԭ�ζ�ʵ��ͬ�к͵ζ����ơ�������������Ҫ��������������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹��������Ũ�ȡ���֪��2KMnO4��5H2O2��6H2SO4===2MnSO4��8H2O��5O2��������д���пհף�
���� (���ʽ����ʽ��)�ζ�����ȡ����������Һ25.00 mL����ƿ�У�������������
�ڵζ�ʱ����������ر���Һע����ʽ�ζ����У�������ر���Һ����ʽ�ζ���ԭ��Ϊ �����ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й�����������ʵ���Ũ��Ϊ ��
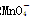 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5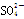 ��3H2O����ջش����⣺
��3H2O����ջش����⣺