��Ŀ����
����Ŀ��ʵ�����ð�����ԭ����ͭ�ķ����ⶨͭ�Ľ������ԭ����������Ӧ�Ļ�ѧ����ʽΪ
2NH3+3CuO![]() N2+3Cu+3H2O���Իش�
N2+3Cu+3H2O���Իش�
(1)���ѡ�òⶨ��Ӧ��CuO��������H2O������[m(CuO)��m(H2O)]ʱ�����������������һ�������鷽����
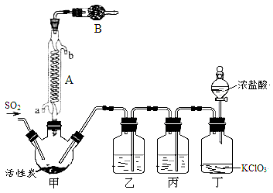
�� �������ӵ�˳��(����ĸ��ű�ʾ���������ظ�ʹ�� )__________��d��Ũ�����������___________��___________________��ʵ�����ʱ�۲쵽a�е�������_____________________��
���г�����Cu�����ԭ�������ı���ʽ__________________��
�����������ʹ�ⶨ���ƫ�����_____________��������ѡ����ղ���1����ȷ�𰸣�������ĸ�����д��
A.CuOδȫ����ԭΪCu B.CuO�ܳ� C.CuO�л���Cu
(2)����Բ�����������װ�ã�����������ѡ�òⶨ����������___________��
A.m (Cu)��m(CuO) B. m (N2)��m (H2O)
C.m (Cu)��m(H2O) D.m(NH3)��m (H2O)
���𰸡� b��c��a��c��d ���ղ����Ķ���NH3 ��ֹ������H2O����C��Ӱ���� ��ɫ��CuOȫ��ת��ɺ�ɫ�Ĺ��� ![]() A��C A��C
A��C A��C
����������1�����������ӵĹؼ����ǣ�bװ�ò�����NH3���л��е�ˮ����Ӧ����C����ʯ�ң���ȥ��ͨ��a��CuO���������ˮ����������NH3��Ӧ��ͨ��C����ʯ�ң�����ˮ��Ȼ����ͨ��d���ն���İ���������ȷ������˳��ӦΪ��b��c��a��c��d��d��Ũ��������������ն����NH3��������ֹ�����е�ˮ�ֽ���c��Ӱ��ⶨ�����ʵ�������a�к�ɫ��CuOȫ��ת��ɺ�ɫ��Cu���ڸ��ݷ���ʽ��֪
H2+CuO![]() Cu+H2O
Cu+H2O
x+16 18
m��CuO�� m��H2O��
Cu�����ԭ������x=![]()
��A����CuOδ��ȫ����ԭ��Cu������ʽ��![]() ��ֵ��ʵ�ʴ��ƫ����A��ȷ��B����CuO�ܳ�����m��CuO��ƫ��m��H2O��Ҳƫ���DZ�С��B������C��
��ֵ��ʵ�ʴ��ƫ����A��ȷ��B����CuO�ܳ�����m��CuO��ƫ��m��H2O��Ҳƫ���DZ�С��B������C��
��CuO�л���Cu����m��CuO��ƫ��m��H2O�����䣬��![]() ƫ���ƫ����C��ȷ����2�����Բ�����������װ�ã�����������ѡ�òⶨ��������������CuO��Cu����ֻ��ѡ��A��m��Cu����m��CuO����C��m��Cu����m��H2O������ѡAC��
ƫ���ƫ����C��ȷ����2�����Բ�����������װ�ã�����������ѡ�òⶨ��������������CuO��Cu����ֻ��ѡ��A��m��Cu����m��CuO����C��m��Cu����m��H2O������ѡAC��