��Ŀ����
���ú����۵����ʿ����������ᣮ������������������̣��������̻ش��������⣺
B���ճ���������������ζ�ij����л������Щ�����к���B��
��1��д����ѧ����ʽ����ע����Ӧ���ͣ�
��B����ᷴӦ��
��B��C��
��2�����֤�������Ѿ�����ˮ��

B���ճ���������������ζ�ij����л������Щ�����к���B��
��1��д����ѧ����ʽ����ע����Ӧ���ͣ�
��B����ᷴӦ��
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
| ||
CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O
��
| ||
����
����
��Ӧ����B��C��
2CH3CH2OH+O2
2CH3CHO+2H2O
| Cu��Ag |
| �� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| Cu��Ag |
| �� |
����
����
��Ӧ����2�����֤�������Ѿ�����ˮ��
ʹ���Ƶ�Cu��OH��2����Һ��죬��ȡһС���ֵ����ˮ����
ʹ���Ƶ�Cu��OH��2����Һ��죬��ȡһС���ֵ����ˮ����
������������������������ˮ�����������ǣ��������ڴ����������������Ҵ����Ҵ��ɱ�����Ϊ��ȩ���������������ᣮ
����⣺��1������������������ˮ�����������ǣ��������ڴ����������������Ҵ����Ҵ��ɱ�����Ϊ��ȩ���������������ᣬ��AΪ�����ǣ�BΪ�Ҵ���CΪ��ȩ��
���Ҵ���������Ũ���������·���������Ӧ����������������Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��ȡ������������
��BΪ�Ҵ���CΪ��ȩ���Ҵ����ڴ��������·���������������ȩ��
��Ӧ�ķ���ʽΪ2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��������
��3��Ϊ֤�����۷�������ˮ�⣬�ɰ�ˮ��Һ�ֳ���֧�Թܣ�����һ֧�Թ��м����ˮ��Һ����һ֧�Թ����ȼ��� NaOH��Һ���ټ��� ����Cu��OH��2��Һ���м��飮
�ʴ�Ϊ��ʹ���Ƶ�Cu��OH��2����Һ��죬��ȡһС���ֵ����ˮ������
���Ҵ���������Ũ���������·���������Ӧ����������������Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH
| ||
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| ||
��BΪ�Ҵ���CΪ��ȩ���Ҵ����ڴ��������·���������������ȩ��
��Ӧ�ķ���ʽΪ2CH3CH2OH+O2
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |
��3��Ϊ֤�����۷�������ˮ�⣬�ɰ�ˮ��Һ�ֳ���֧�Թܣ�����һ֧�Թ��м����ˮ��Һ����һ֧�Թ����ȼ��� NaOH��Һ���ټ��� ����Cu��OH��2��Һ���м��飮
�ʴ�Ϊ��ʹ���Ƶ�Cu��OH��2����Һ��죬��ȡһС���ֵ����ˮ������
���������⿼����۵�ˮ�⡢�л���ĺϳ��Լ�����ˮ��̶ȵļ��飬��Ŀ�Ѷ��еȣ�ע�ⳣ���л�������ʣ�

��ϰ��ϵ�д�
�����Ŀ

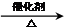 2CH3CHO+2H2O
2CH3CHO+2H2O
