��Ŀ����
����Ŀ��CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��Co(OH)3����������Fe2O3��Al2O3��MnO��)��ȡCoCl2��6H2O�Ĺ����������£�

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�����������£�ClO3����������Co2+��ClO3��ת��ΪCl����
�۲���������������������ʽ����ʱ��Һ��pH���±���(��������Ũ��Ϊ��0.01mol/L)

��CoCl2��6H2O�۵�Ϊ86�棬������110~120��ʱ��ʧȥ�ᾧ������ˮ�Ȼ��ܡ�
��ش�
(1)д������������Co2O3������Ӧ�����ӷ���ʽ___________��
(2)�����Һ�м��� NaClO3������Ҫ��Ӧ�����ӷ���ʽ___________��
(3)����Na2CO3��pH��5.2�����������õ��ij������ɷ�Ϊ___________��
(4)��ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ��ʾ����ȡ��ʹ�õ�����pH��Χ��___________��(��ѡ�������ĸ)

A 1.0~2.0 B 2.5~3.5 C 4.0~5.0
(5)�Ƶõ�CoCl2��6H2O�ں��ʱ���ѹ��ɵ�ԭ����___________��
(6)Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O��������ȡ2g�Ĵֲ�Ʒ����ˮ�����100mL��Һ��ȡ��20mL������ƿ������K2CrO4��ָʾ��( Ag2CrO4Ϊש��ɫ����)����0.2mol/L��AgNO3��Һ�ζ����յ㣬�ظ�2-3�Σ�ƽ������AgNO3����Һ10.00mL���ôֲ�Ʒ��CoCl2��6H2O����������Ϊ___________����K2CrO4��ָʾ��ʱ����Ҫ������ҺpHֵΪ6.5~10.5���Է���ԭ��______________________��
���𰸡�Co2O3 + SO32- +4H+ = 2Co2+ + SO42- +2H2O 6H+ + 6Fe2+ + ClO3- = 6Fe3+ + Cl- + 3H2O Fe(OH)3, Al(OH)3 B ���ͺ���¶ȣ���CoCl2��6H2O ������ʧȥ�ᾧˮ 59.5% pH̫СK2CrO4����Cl-(��ת��ΪCr2O72-)�� pH̫������Ag(OH)���� (��Ag2O����)
��������
���ܷ����м�������������ƣ��ɵ�CoCl2��AlCl3��FeCl2������NaClO3���ɵõ�FeCl3��Ȼ�����Na2CO3��pH��5.2���ɵõ�Fe��OH��3��Al��OH��3���������˺�������Һ��Ҫ����CoCl2��Ϊ�õ�CoCl26H2O���壬Ӧ�����¶���86�����£�����ʱҪ��ֹ�¶ȹ��߶�ʧȥ�ᾧˮ���ɼ�ѹ��ɣ�
��1����������ͼ�����Ϣ����Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�Ƚ��н��
��2��NaClO3�������ǽ�Fe2+������Fe3+��
��3�����������ӡ�����������̼������ӷ���˫ˮ�����ɳ����Ͷ�����̼���н��
��4���ɱ������ݿ�֪��������ҺPH��3.0��3.5֮�䣬��ʹMn2+��ȫ����������ֹCo2+ת��ΪCo��OH��2������
��5���¶ȸ�ʱCoCl26H2O�ֽ⣻
��6������CoCl2��6H2O��AgNO3��Ӧ�Ĺ�ϵʽ����CoCl2��6H2O�Ĵ��ȣ�pH̫СK2CrO4����Cl-(��ת��ΪCr2O72-)�� pH̫������AgOH������
��1��ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��Co��OH��3������������������ƣ�����Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�����Co2O3���������������������·���������ԭ�����ݵ���غ�͵�ʧ�����غ㣬��Ӧ�����ӷ���ʽΪ��Co2O3+SO32-+4H+=2Co2++SO42-+2H2O��
��2��NaClO3�������ǽ�Fe2+������Fe3+���䷴Ӧ�����ӷ���ʽΪ��ClO3-+6Fe2++6H+=Cl-+6Fe3++3H2O��
��3��NaClO3�������ǽ�Fe2+������Fe3+����Na2CO3��pH��5.2����������̼������ӷ���˫ˮ���������������Ͷ�����̼��ˮ������ӷ���ʽΪ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2��������������̼������ӷ���˫ˮ���������������Ͷ�����̼��ˮ������ӷ���ʽΪ��2Fe3++3CO32-+3H2O=2Fe��OH��3��+3CO2�������Գ���X�ijɷ�Ϊ��Fe��OH��3��Al��OH��3��
��4����������ͼ��֪����ʱ��Һ�д���Mn2+��Co2+�������ӣ�����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��֪��������ҺpH��3.0��3.5֮�䣬��ʹMn2+��ȫ����������ֹCo2+ת��ΪCo��OH��2��������ѡB��
��5����������֪��CoCl26H2O�������ȶ�����������110��120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܣ�Ϊ��ֹ��ֽ⣬�Ƶõ�CoCl26H2O�轵�ͺ���¶ȣ�
��6��CoCl2��6H2O��2AgNO3
238g 2mol
xg 0.2mol/L��0.01L
![]()
X=0.238g
CoCl2��6H2O�Ĵ���Ϊ![]() 59.5%
59.5%
pH̫СK2CrO4����Cl-(��ת��ΪCr2O72-)�� pH̫������AgOH������������Ҫ������ҺpHֵΪ6.5��10.5��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ҵˮ���·��Ʊ��������ƵĹ���������ͼ��ʾ:
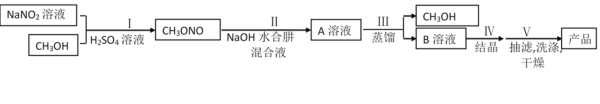
һ���ϳ�ˮ����
��֪��![]() (ˮ����)��ˮ���ܣ��ж��Ҳ��ȶ�,100�������ֽ�ʧˮ������ǿ��ԭ�Ժ�ǿ���ԡ�
(ˮ����)��ˮ���ܣ��ж��Ҳ��ȶ�,100�������ֽ�ʧˮ������ǿ��ԭ�Ժ�ǿ���ԡ�![]() (ˮ����)�۵�-40��,�е�118.5�档
(ˮ����)�۵�-40��,�е�118.5�档
�ϳ�![]() ��װ����ͼ1��ʾ��
��װ����ͼ1��ʾ��![]() ������Һ������
������Һ������![]() ˮ��Һ��40�����·�Ӧһ��ʱ�����Ѹ��������110�������Ӧ�����Ƶ�ˮ���¡�
ˮ��Һ��40�����·�Ӧһ��ʱ�����Ѹ��������110�������Ӧ�����Ƶ�ˮ���¡�
(1)д��![]() �Ľṹʽ____________��
�Ľṹʽ____________��![]() �ĵ���ʽ____________
�ĵ���ʽ____________
(2)��д����ȡ![]() �����ӷ���ʽ___________________________
�����ӷ���ʽ___________________________
��ʵ����ͨ����Һ©���μӵ���Һ��_________������_______________ͼ1ʹ�������ܵ�Ŀ����____________________________________
�۴ӷ�Ӧ��Ļ���ܷ����![]() ��Ӧ�ò��õķ��뷽�����������________��
��Ӧ�ò��õķ��뷽�����������________��
�����ϳɵ�������(![]() )����
)����
��֪��������ʵ������������±�
�۵�� | �е�� | �ܽ��� | |
| -97 | 67.1 | ��ˮ���� |
ˮ����( | -40 | 118.5 | ��ˮ�������ܣ����������Ѻ��ȷ� |
���������( | -17 | -12 | �����Ҵ������� |
NaN3 | ��ˮ���ܣ����������ѡ������Ҵ� |
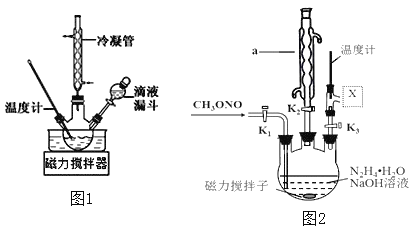
(3)ʵ����ģ������̲�����ʵ��װ����ͼ2
�ٸ���ʵ�鷢���¶���20�����ҷ�Ӧ��ѡ���Ժ�ת������ߣ����Ǹ÷�Ӧ���ڷ��ȷ�Ӧ����˿��Բ�ȡ�Ĵ�ʩ��________________��(д��1�㼴��)
��ͼ��![]() �����ӵ������װ��ӦΪ��ͼ�е�__________��
�����ӵ������װ��ӦΪ��ͼ�е�__________��
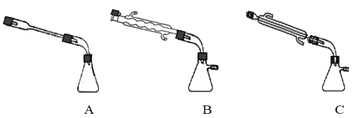
�۲�������Ʊ��������ƵIJ�����________(�����)���������Һ![]() ��������ĺ�������˳����_______��(�����)
��������ĺ�������˳����_______��(�����)
�ٴ�![]() ���ر�
���ر�![]() �ڴ�
�ڴ�![]() �ۼ��� �ܹر�
�ۼ��� �ܹر�![]()
(4)���������Һ![]() ������������Һ�����
������������Һ�����![]() ,
,![]() �ᾧ�����������������________ϴ�Ӿ��塣
�ᾧ�����������������________ϴ�Ӿ��塣
A.ˮ B.���� C.�Ҵ�ˮ��Һ D.�Ҵ�
(5)��ҵ�ϻ�������ˮ���»�ԭ����ȡ�⻯�ƣ�����������ͼ��
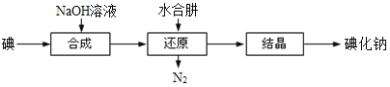
��ԭ�����п������ƻ���м���������ˮ���£���ˮ���»�ԭ���ƵõIJ�Ʒ���ȸ��ߣ�ԭ����______________________________________��







