��Ŀ����
�����12�֣�
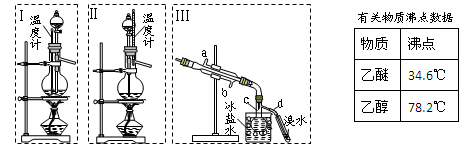
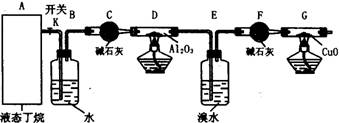
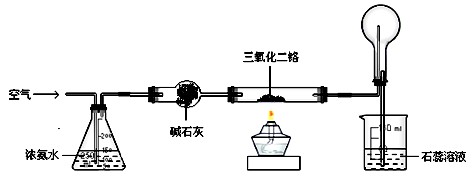
��ͼ��ģ�ҵ������ԭ����ʵ��װ�á����þƾ��ƶ�Ӳ�ʲ���������������������ȣ�Ȼ��ѿ�������ʢ��Ũ��ˮ����ƿ�����������������ֺ���״̬ʱ����ȥ�ƾ��ơ���������ش�
1��Ӳ�ʲ����������Ӧ�Ļ�ѧ����ʽΪ ��
����������������ʱ��Ҫ��ȥ�ƾ��ƣ�ԭ���ǣ� ��
2��Բ����ƿ���ʵ�������� ����������������� ��
3��ʵ������У���������ع��������ʯ����Һ����ɫ ��
4��ʵ���������Բ����ƿ�ڱ�����ʱ�ῴ��������ɫ���壬д�����ɸþ���Ļ�ѧ����ʽ�� ��
5�����Ƶ�������������Ч���Ϻá�ʵ����һ���ü��ȷֽ�(NH4)2Cr2O7�ķ�������������������Ӧ�Ļ�ѧ����ʽΪ ��
6����ҵ��Ҫ���Ũ�Ƚϴ�����ᣬ������ϡ�����м�����ˮ������þ��Ũ���ᣬȻ�� ����һ�ֲ������ƣ���ʵ�����ﱣ��Ũ����ķ����� ��
����12�֣�
1��4NH3 + 5O2 4NO + 6H2O��1�֣�
4NO + 6H2O��1�֣�
�÷�Ӧ�Ƿ��ȷ�Ӧ���¶�̫�߿���ʹ�����Ļ��Խ��ͣ����������ѣ��������֣���1�֣�
2���к���ɫ���������1�֣� �ṩO2���Ҵٽ����Ļӷ���2�֣�
3�����ɫ��1�֣�
4��NH3 + HNO3 �� NH4NO3��1�֣�
5��(NH4)2Cr2O7 Cr2O3 + N2�� + 4H2O��2�֣�
Cr2O3 + N2�� + 4H2O��2�֣�
6������1�֣� ʢ����ɫƿ���ܷ������������2�֣�
����