��Ŀ����
|
��֪���� Cu(s)��H2O2(l)+2H+ (aq)��Cu2+(aq)��2H2O(l) ��H 1 ��2H2O(l)��2H2(g)��O2(g) ��H 2 ��2H2O2(l)��2H2O(l)��O2 (g) ��H 3 ��Cu(s)��2H+ (aq)��Cu2+(aq)��H2 (g) ��H4 ��H4����ȷ����ʽΪ�� ��
|
B
���ݸ�˹���ɣ��ܣ��٣� ���ڡ�
���ڡ� ���ۣ����H4����H1��
���ۣ����H4����H1�� ����H2��
����H2�� ����H3��������ȷ����B��
����H3��������ȷ����B��
�йظ�˹���ɵ���ؼ��㣬�����Ǹ߿����ȵ㣬�����Ƿ�ѡ�����е��ʱ�ļ��㻹��ѡ�������ʱ�ļ��㶼�벻����˹���ɵĿ��顣
 ���ڡ�
���ڡ� ���ۣ����H4����H1��
���ۣ����H4����H1�� ����H2��
����H2�� ����H3��������ȷ����B��
����H3��������ȷ����B���йظ�˹���ɵ���ؼ��㣬�����Ǹ߿����ȵ㣬�����Ƿ�ѡ�����е��ʱ�ļ��㻹��ѡ�������ʱ�ļ��㶼�벻����˹���ɵĿ��顣

��ϰ��ϵ�д�
�����Ŀ
 N2��g��+CO2��g��ij�о�С��������ܱ������м���һ�����Ļ���̿��NO�����£�T�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+CO2��g��ij�о�С��������ܱ������м���һ�����Ļ���̿��NO�����£�T�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�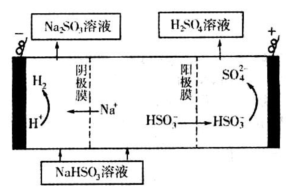
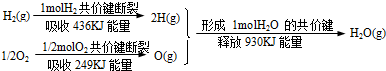
 2SO3(g) ��H1 ƽ�ⳣ��ΪK1
2SO3(g) ��H1 ƽ�ⳣ��ΪK1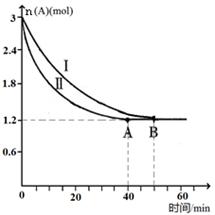
 CH3OH(g)����H1����90 kJ��mol��1
CH3OH(g)����H1����90 kJ��mol��1
 CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________(��Ѿ�����û�С�)�ﵽƽ�⣬ԭ����_________________________________________________
CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________(��Ѿ�����û�С�)�ﵽƽ�⣬ԭ����_________________________________________________ CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���

