��Ŀ����
11����þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϣ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ3%-5%����þ�Ͻ𣨲�������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհף�[̽��һ]
ʵ�鷽������þ�Ͻ�$\stackrel{NaOH��Һ}{��}$�ⶨʣ���������
ʵ���з�����Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
ʵ�鲽�裺
��1����ȡ5.4g��þ�Ͻ��ĩ��Ʒ��Ͷ��V mL 2.0mol•L-1NaOH��Һ�У���ַ�Ӧ��NaOH��Һ�����V��100mL��
��2�����ˡ�ϴ�ӡ�����������壮�ò�������δϴ�ӹ��壬���þ������������ƫ�ߣ��ƫ�ߡ���ƫ�͡���
[̽����]
ʵ�鷽������þ�Ͻ�$\stackrel{����}{��}$�ⶨ������������ʵ��װ�ã�
�������ۣ�
��1��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�ã��������ǣ�����Ҫ�����Ҫ������Ҫ����
��2��Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע��������ǣ�д�����㣩���ټ��װ�õ������ԣ��ںϽ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����Ⱥ����𰸣���
[̽����]
ʵ�鷽��������xg��þ�Ͻ��ĩ����������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������գ�
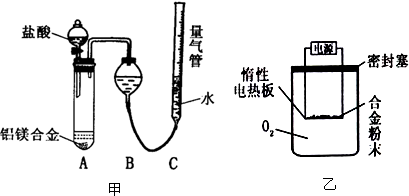
�������ۣ�
��1��������Mg��������������ʵ���л���ⶨ�����������պ���������
��2�����ÿ�������O2����ʵ�飬�Բⶨ�����Ӱ�죿ƫ�ߣ��ƫ�ߡ���ƫ�͡�����Ӱ�족��
���� ̽��һ����������������Һ��Ӧ����ƫ��������������
��1��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
̽��������1���Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã�
��2��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�������Ȼ�Ӱ��ⶨ�����
̽��������1��Mg��Al���ܹ���������ѧ��Ӧ���������
��2���ÿ�������O2����ʵ�飬������Ӧ��3Mg+N2$\frac{\underline{\;��ȼ\;}}{\;}$Mg3N2��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C���ⶨ���ɹ�����������
��� �⣺̽��һ����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��1����þΪ��ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 2mol
5.4g V��10-3L��2.0mol/L
����54g��5.4g=2mol����V��10-3L��2.0mol/L����
��ã�V=100mL����V��NaOH��Һ����100mL��
�ʴ�Ϊ��100mL��
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
̽��������1�������Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã�
�ʴ�Ϊ������Ҫ��
��2����Ӧ��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽⶼ��Ӱ��ⶨ�����
�ʴ�Ϊ�����װ�õ������ԣ��Ͻ���ȫ�ܽ⣨������������ᣬ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����Ⱥ����𰸣���
��̽������
��1��Mg��Al����������Ӧ�����ɽ������������ⶨ�������������
�ʴ�Ϊ�����պ�����������
���ÿ�������O2����ʵ�飬������Ӧ��3Mg+N2$\frac{\underline{\;��ȼ\;}}{\;}$Mg3N2��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C���ⶨ���ɹ�������������þ����������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�| A�� | ������ | B�� | ��ԭ�� | C�� | ���� | D�� | ���� |
| A�� | C2H4��C2H4O�����Ժ��ֱ�����ϣ�ֻҪ��������������䣬��ȫȼ��ʱ����ˮ������Ҳ���� | |
| B�� | ���������Ľṹ��ʽ��HC-O-OC2H5 | |
| C�� | ���͡����͡�ֲ���ͺ��Ͷ���̼�⻯���� | |
| D�� | HCOOCH3��CH3OCHO��ʾͬһ������ |
| A�� | ��������ƿ��ʢԼ$\frac{1}{3}$���������ˮ�������뼸����ʯ | |
| B�� | ���¶ȼ�ˮ������֧�ܿڱ���ˮƽ | |
| C�� | ��ˮ���������¿ڳ����Ͽ��� | |
| D�� | �ռ�����Һ��ȡ����������������ϡ���ᣬ���������� |
| A�� | ��FeCl3��Һ��ʴӡˢ��·����ͭ����Fe3++Cu�TFe2++Cu2+ | |
| B�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| C�� | ��С�մ�����θ����ࣺCO${\;}_{3}^{2-}$+2H+�TCO2��+H2O | |
| D�� | ƫ��������Һ��ͨ�����CO2��AlO${\;}_{2}^{-}$+CO2+2H2O�TAl��OH��3��+HCO${\;}_{3}^{-}$ |
 ��100mL FeI2��Һ����ͨ��Cl2������������I2��Fe3+��IO3-������Fe3+��I2�����ʵ�����n��Cl2���ı仯��ͼ��ʾ����ش��������⣺
��100mL FeI2��Һ����ͨ��Cl2������������I2��Fe3+��IO3-������Fe3+��I2�����ʵ�����n��Cl2���ı仯��ͼ��ʾ����ش��������⣺