��Ŀ����
������ˮ�����������������ؾ���K3[Fe(C2O4)3]��3H2O��������Ӱ����ɫӡˢ������мΪԭ�ϵ��Ʊ��������£�
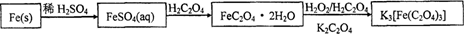
��ش��������⣺
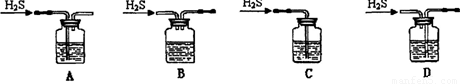
��1����м�г�����Ԫ�أ���ϡ����ʱ������ж���H2S���壬��������������Һ���գ���������װ����ȷ���� ��
��2���Ƶõ�FeSO4��Һ�������������H2SO4�ữ��Ŀ���� ����Ҫ����Һ�еõ��̷�FeSO4��7H2O��������е�ʵ������� ����˳����д����
a������ϴ�� b������Ũ�� c����ȴ�ᾧ d������ e������
��3���þ�������110�����ȫʧȥ�ᾧˮ�����������¶ȿɷ����ֽⷴӦ��
�ٷֽ�õ����������������װ�ý���ʵ��
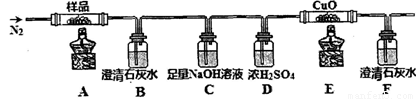
װ�ü�������Ժ���ͨһ��ʱ��N2����Ŀ��Ϊ ������ʵ��ʱ��Ϩ��ƾ�����ͨ��N2�����£���Ŀ��Ϊ ��ʵ������й۲쵽B��F�г���ʯ��ˮ������ǣ�E���к�ɫ�������ɣ������������ ��
�ڷֽ�õ��Ĺ�����ﺬ��K2CO3��FeO��Fe����ˮ�ܽ⡢���ˡ�ϴ�ӡ�����õ�������Ʒ���������������ʵ�鷽���Ը���Ʒ�������ʺ����ⶨ��
���ס�a g��Ʒ ��Һ
��Һ
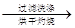 �ù���b g
�ù���b g
���ҡ�a g��Ʒ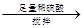
 ��������������Va mL
��������������Va mL
������a g��Ʒ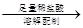 250 mL��Һ
250 mL��Һ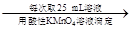 ����ƽ������0.1 mol��L��1����KMnO4��ҺVb
mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ�������
��
����ƽ������0.1 mol��L��1����KMnO4��ҺVb
mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ�������
��
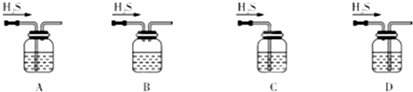
��1��A ��2����ֹFe2����ˮ�� b c a e
��3�����ų�װ���еĿ����� ��ֹ����ʵ���� ��ֹҺ������������� CO2��CO
�ڱ����� ʹ�������ܽ���Ʒ��������Ҳ�ɱ�����MnO4-����
��������
�����������1��������������ʱ�����ܵ����ӷ�ʽ�dz����̳������ҳ��ĵ�����Ҫ��û��Һ���£��̵ĵ�������¶���������ɡ���ѡ��ΪA����2���Ƶõ�FeSO4��Һ�������������H2SO4�ữ��Ŀ���Ƿ�ֹFe2����ˮ���ʹ��Һ����ǡ���Ҫ����Һ�еõ��̷�FeSO4��7H2O��������е�ʵ�����������Ũ������ȴ�ᾧ������ϴ�ӡ������ѡ��Ϊ b c a e����3����װ�ü�������Ժ�Ҫ��ͨһ��ʱ��N2����Ŀ��Ϊ���ų�װ���еĿ����� ��ֹ����ʵ������ʵ������й۲쵽B�г���ʯ��ˮ�����˵������CO2��E���к�ɫ�������ɣ�F�еij���ʯ��ˮ�������˵��������ﺬ��CO���������������CO2��CO���ڷֽ�õ��Ĺ�����ﺬ��K2CO3��FeO��Fe����ˮ�ܽ⡢���ˡ�ϴ�ӡ�����õ�������Ʒ���ף���Ʒag,���������������ܽ�õ�Fe(NO3)3������Һ�м���������NaOH��Һ�õ�Fe(OH)3������������˳���ϴ�Ӹɾ���Ȼ�����գ�������Ӧ��2Fe(OH)3 Fe2O3+3H2O.��ɵõ��Ĺ�����������Fe2O3�����������������FeԪ�ص�����������ٸ��ݺ�����Ʒ��������Fe���������dz�����������ֵĺ�������ȷ���ң�������Ʒ�м���������ϡ���ᷢ����Ӧ��Fe+H2SO4=FeSO4+H2�������ݷų�������������ɼ����Fe���������̶����FeO��������������Ʒ�и���ֵĺ����õ���⡣��ȷ��������Ʒ�������������ܽ�ʱ������Ӧ��Fe+2HCl=FeCl2+H2��;FeO+2HCl=FeCl2+H2O.ȡ25ml����Һ������KMnO4��Һ�ζ�ʱFe2+��Cl-�������Ը��������������˲��ܸ������ĵĸ��������Һ�����ʵ����Ķ�����ȷ����Һ��Fe2+�Ķ��١��ʲ���ȷ����Ʒ�и���ֺ����Ķ��١���������������ַ����б���ȷ�����ʵ���ɡ�
Fe2O3+3H2O.��ɵõ��Ĺ�����������Fe2O3�����������������FeԪ�ص�����������ٸ��ݺ�����Ʒ��������Fe���������dz�����������ֵĺ�������ȷ���ң�������Ʒ�м���������ϡ���ᷢ����Ӧ��Fe+H2SO4=FeSO4+H2�������ݷų�������������ɼ����Fe���������̶����FeO��������������Ʒ�и���ֵĺ����õ���⡣��ȷ��������Ʒ�������������ܽ�ʱ������Ӧ��Fe+2HCl=FeCl2+H2��;FeO+2HCl=FeCl2+H2O.ȡ25ml����Һ������KMnO4��Һ�ζ�ʱFe2+��Cl-�������Ը��������������˲��ܸ������ĵĸ��������Һ�����ʵ����Ķ�����ȷ����Һ��Fe2+�Ķ��١��ʲ���ȷ����Ʒ�и���ֺ����Ķ��١���������������ַ����б���ȷ�����ʵ���ɡ�
���㣺�������װ�õ�ѡ���������ʵ�鲽�輰������и���ֺ����IJⶨ������֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ͼ��ʾװ�õļ�ѹ������ĸҺ���룮
ͼ��ʾװ�õļ�ѹ������ĸҺ���룮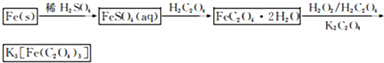



 �ɱ�����KMnO4��Һ�����ų�CO2���壬����ʵ�������K3[Fe(C2O4)3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
�ɱ�����KMnO4��Һ�����ų�CO2���壬����ʵ�������K3[Fe(C2O4)3]��3H2O�����ⶨ����KMnO4����Һ�ζ���