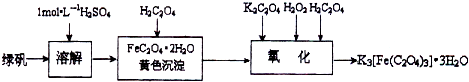
��Ŀ����
K3[Fe��C2O4��3]?3H2O[���������������ؾ���]������ˮ���������Ҵ�������Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�����ط�Ӧ�������£���ش��������⣺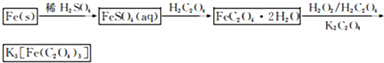
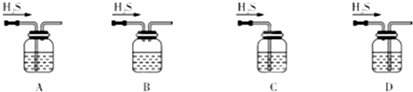
��1����м�г�����Ԫ�أ�������Ʊ�FeSO4ʱ������ж���H2S���壬�������������������Һ���գ���������װ����ȷ����
��2���ڵõ���FeSO4��Һ�������������H2SO4�ữ��Ŀ����
��3�������������ᾧˮ��ͨ�������������ⶨ����Ҫ�����У��ٳ����������ں������ѽᾧˮ������ȴ���ܳ��������ظ��ڡ��������أ����㣮����ݵ�Ŀ����
��4��C2O42-�ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]?3H2O�����ⶨ����KMnO4����Һ�ζ���
��д���ζ������з�����Ӧ�����ӷ���ʽ
�����еζ�������ʹ�ζ����ƫ�ߵ���
A���ζ���������ˮϴ�Ӻ�����װ���Һ
B����ƿ��װ����Һǰδ�ô���Һ��ϴ
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ���
��ȡ����10.0g���100mL��Һ������ȡ��20mL����ƿ�У���Ũ��Ϊ0.1mol?L-l������KMnO4��Һ�ζ����ﵽ�ζ��յ�ʱ��������KMnO4��Һ24.00mL���������K3[Fe��C2O4��3]?3H2O����������Ϊ
��������1����������װ���м�Ҫ�������������ų�����Ӧ�����壻
��2������������ˮ����������������������������ˮ�⣻�¶ȸ�ʱ��˫��ˮ��ˮ�⣻������������ԭ��������
��3������ݵ��Ǽ��龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ��
��4����C2O42-�ɱ�����KMnO4��Һ����ΪCO2���壬����ԭ���غ��ԭ���غ���ƽ��д���ӷ���ʽ��
�����ݵζ��������ķ����жϣ������Թ��Ϊ��Һ��������ı仯������c�����⣩=
��
A����ʽ�ζ���Ҫ�ñ�Һ��ϴ��
B����ƿ�ô���Һ��ϴ���������ı�Һ�������
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ����ȡ����Һ�������
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ�������ȡ����Һ�����С��
�����ݵζ�ʵ������ӷ���ʽ������ϵ�������������ص����ʵ���������õ�����������
��2������������ˮ����������������������������ˮ�⣻�¶ȸ�ʱ��˫��ˮ��ˮ�⣻������������ԭ��������
��3������ݵ��Ǽ��龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ��
��4����C2O42-�ɱ�����KMnO4��Һ����ΪCO2���壬����ԭ���غ��ԭ���غ���ƽ��д���ӷ���ʽ��
�����ݵζ��������ķ����жϣ������Թ��Ϊ��Һ��������ı仯������c�����⣩=
| c(��)V(��) |
| V(����) |
A����ʽ�ζ���Ҫ�ñ�Һ��ϴ��
B����ƿ�ô���Һ��ϴ���������ı�Һ�������
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ����ȡ����Һ�������
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ�������ȡ����Һ�����С��
�����ݵζ�ʵ������ӷ���ʽ������ϵ�������������ص����ʵ���������õ�����������
����⣺��1��A����װ��������������������Һ�Ӵ�����Ӷ�ʹ�������ս���ȫ���Ҹ�װ������ѹ���ȶ�����������ȫ���⣬��A��ȷ��
B���������������ƽӴ������С���������ղ���ȫ����B����
C��û������װ�ã����¸�װ������ѹ�����������ȫ�¹ʣ���C����
D����װ����Ӧ��ѭ�������̳�����ԭ������D����
��ѡA��
��2������������ˮ���������ѹ�����ԣ�����ϡ������������������ˮ�⣻������������ԭ��֪�����������������Ҵ����ܽ��С�����Կ������Ҵ�ʹ������������������
�ʴ�Ϊ����ֹFe2+��ˮ�⣻���������������Ҵ����ܽ��С�����ھ���������
��3������ݵ��Ǽ��龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ���ʴ�Ϊ�����龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ��
��4����C2O42-�ɱ�����KMnO4��Һ����ΪCO2���壬����ԭ���غ��ԭ���غ��֪���ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
����c�����⣩=
��֪��
A����ʽ�ζ���Ҫ�ñ�Һ��ϴ���ζ���������ˮϴ�Ӻ�����װ���Һ����ҺŨ�ȼ�С�����ı�Һ������ⶨ���ƫ�ߣ���A���ϣ�
B����ƿ�ô���Һ��ϴ���������ı�Һ���������ƿ��װ����Һǰδ�ô���Һ��ϴ������ʵ��Ҫ��B�����ϣ�
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ����ȡ����Һ������ⶨ���ƫ�ߣ���C���ϣ�
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ�������ȡ����Һ�����С���ⶨ���ƫС����D�����ϣ�
�ʴ�Ϊ��AC��
��5��ȡ����10.0g���100mL��Һ������ȡ��20mL����ƿ�У���Ũ��Ϊ0.1mol?L-l������KMnO4��Һ�ζ����ﵽ�ζ��յ�ʱ��������KMnO4��Һ24.00mL���������ӷ���ʽ������ϵ�������㣬����������������Һ��Ũ��Ϊx��
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
2 5
0.1mol?L-l��24.00ml x��20.00ml
x=0.300mol/L
100ml��Һ��K3[Fe��C2O4��3]?3H2O���ʵ���=0.300mol/L��0.1L��
=0.01mol��
�������K3[Fe��C2O4��3]?3H2O����������=
��100%=49.1%��
�ʴ�Ϊ��49.1%��
B���������������ƽӴ������С���������ղ���ȫ����B����
C��û������װ�ã����¸�װ������ѹ�����������ȫ�¹ʣ���C����
D����װ����Ӧ��ѭ�������̳�����ԭ������D����
��ѡA��
��2������������ˮ���������ѹ�����ԣ�����ϡ������������������ˮ�⣻������������ԭ��֪�����������������Ҵ����ܽ��С�����Կ������Ҵ�ʹ������������������
�ʴ�Ϊ����ֹFe2+��ˮ�⣻���������������Ҵ����ܽ��С�����ھ���������
��3������ݵ��Ǽ��龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ���ʴ�Ϊ�����龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ��
��4����C2O42-�ɱ�����KMnO4��Һ����ΪCO2���壬����ԭ���غ��ԭ���غ��֪���ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
����c�����⣩=
| c(��)V(��) |
| V(����) |
A����ʽ�ζ���Ҫ�ñ�Һ��ϴ���ζ���������ˮϴ�Ӻ�����װ���Һ����ҺŨ�ȼ�С�����ı�Һ������ⶨ���ƫ�ߣ���A���ϣ�
B����ƿ�ô���Һ��ϴ���������ı�Һ���������ƿ��װ����Һǰδ�ô���Һ��ϴ������ʵ��Ҫ��B�����ϣ�
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ����ȡ����Һ������ⶨ���ƫ�ߣ���C���ϣ�
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ�������ȡ����Һ�����С���ⶨ���ƫС����D�����ϣ�
�ʴ�Ϊ��AC��
��5��ȡ����10.0g���100mL��Һ������ȡ��20mL����ƿ�У���Ũ��Ϊ0.1mol?L-l������KMnO4��Һ�ζ����ﵽ�ζ��յ�ʱ��������KMnO4��Һ24.00mL���������ӷ���ʽ������ϵ�������㣬����������������Һ��Ũ��Ϊx��
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
2 5
0.1mol?L-l��24.00ml x��20.00ml
x=0.300mol/L
100ml��Һ��K3[Fe��C2O4��3]?3H2O���ʵ���=0.300mol/L��0.1L��
| 1 |
| 3 |
�������K3[Fe��C2O4��3]?3H2O����������=
| 0.01mol��491g/mol |
| 10.0g |
�ʴ�Ϊ��49.1%��
���������⿼�����������ʵ�ʵ��̽����ʵ�鷽���������������㣬��Ҫ�ǵζ�ʵ��Ĺ��̷����������������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ

 ͼ��ʾװ�õļ�ѹ������ĸҺ���룮
ͼ��ʾװ�õļ�ѹ������ĸҺ���룮