��Ŀ����
����Ŀ������������(NOSO4H)��ƷΪ���νᾧ���������ᣬ��ˮ�ֽ⣬��������Ⱦ�ϡ�SO2��Ũ������Ũ�������ʱ���Ʊ�NOSO4H����Ӧԭ��Ϊ��SO2+HNO3��SO3+HNO2��SO3+HNO2��NOSO4H��
(1)����������(NOSO4H)���Ʊ���
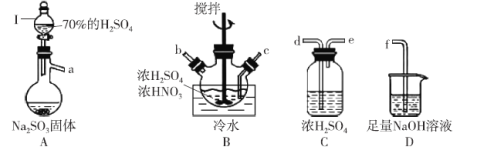
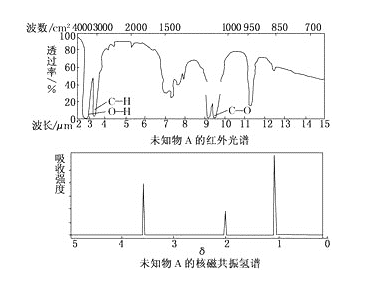
�ٴ�Һ©��I�е���������Һ�岻�µΣ����ܵ�ԭ����_______��
�ڰ����������ҵ�˳����������������˳��Ϊ_______ (�������ӿ���ĸ�������������ظ�ʹ��)��
��A�з�Ӧ�ķ���ʽΪ_______��
��B������ˮ�����¶�һ�������20��C���¶Ȳ�������͵�ԭ��Ϊ_______��
(2)����������(NOSO4H)���ȵIJⶨ����ȡ1.500g��Ʒ����250 mL�ĵ���ƿ�У�������100.00 mLŨ��Ϊ0.1000 mol��L��1��KMnO4����Һ��10 mL25%��H2SO4��ҡ�ȣ���0.5000 mol��L��1 Na2C2O4����Һ�ζ����ζ�ǰ����1.02 mL������ζ��յ�ʱ����Ϊ31.02 mL��
��֪��
i.__KMnO4��__NOSO4H��__��__K2SO4��__MnSO4��__HNO3��__H2SO4
ii.2KMnO4��5Na2C2O4��8H2SO4��2MnSO4��10CO2����8H2O
����ɷ�Ӧi�Ļ�ѧ����ʽ��_______KMnO4+_______NOSO4H+_______��_______K2SO4+_______MnSO4+_______HNO3+_______H2SO4
�ڵζ��յ������Ϊ_______��
�۲�Ʒ�Ĵ���Ϊ_______��������3λ��Ч���֣�
���𰸡���Һ©���Ͽڵ�����δ��©��δ�������ͨ��©�����ӵİ���δ�뾱����ͨ���� a��de��cb��de��f Na2SO3+H2SO4=Na2SO4+H2O+SO2����Na2SO3+2H2SO4=2NaHSO4+ SO2�� �¶�̫�ͣ���Ӧ����̫�����¶�̫�ߣ������ֽ⣬SO2�ݳ� 2 5 2H2O 1 2 5 2 �������һ��Na2C2O4��Һ��,��Һ��dz��ɫ��Ϊ��ɫ�Ұ������ɫ���ָ� 84.7%
��������
��1����©���ڵ�Һ���������ͨʱҺ�����˳�����£�
������������(NOSO4H)��ˮ�ֽ⣬װ��A��ȡ��SO2�к���ˮ�����������ȸ�����ͨ��B�з�Ӧ��ȡ����������(NOSO4H)��ͬʱ��Ҫ��ֹ�����л�����ʵ�������е�ˮ��������B�У�SO2�ж���δ��Ӧ��ʣ��SO2�����ŷŵ������У��ݴ˷�����
���������������Ʒ�Ӧ���������ƣ����������ƣ��Ͷ�������
���¶�Ӱ�췴Ӧ���ʣ�ͬʱŨ����ȶ���
��2���ٸ���������ԭ��Ӧ�����ϼ����������ӵ�ʧ���غ㼰�����غ������ƽ��
����������ԭ�ζ������У�������������Ϻ�ɫ������������ԭ��Ӧʱ��ɫ��ȥ�����������ζ�������ָʾ����
�۽�Ϸ�Ӧii������������ݼ��������KMnO4�������ڽ�Ϸ�Ӧ����NOSO4H�����ʵ��������������Ʒ�Ĵ��ȡ�
(1)
�ٷ�Һ©���Ͽ�����δ��©��δ�������ͨ��©�����ӵİ���δ�뾱����ͨ���������ɵ���Һ�岻�µΣ�
��Ϊ��Һ©���Ͽ�����δ��©��δ�������ͨ��©�����ӵİ���δ�뾱����ͨ������
��Aװ�õ�Ŀ��Ϊ�Ʊ�SO2���壬������SO2����HNO3��Ӧ�Ʊ�NOSO4H����NOSO4H��ˮ�⣬����SO2���ȸ����SO2����Ⱦ�������Ʊ���������β����������װ������˳��ΪA��C��B��C��D����a��de��cb��de��f��
�ʴ�Ϊa��de��cb��de��f��
��Aװ�õ�Ŀ��Ϊ�Ʊ�SO2���壬����ʽΪ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Na2SO3+2H2SO4��Ũ��=2NaHSO4+H2O+SO2����
�ʴ�ΪNa2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Na2SO3+2H2SO4��Ũ��=2NaHSO4+H2O+SO2����
��B�С���ˮ�����¶�һ�������20�棬�¶Ȳ��˹�����͵�ԭ��Ϊ�¶ȹ��ߣ�HNO3�ֽ⣬SO2�ݳ����¶ȹ��ͣ���Ӧ����̫����
�ʴ�Ϊ�¶ȹ��ߣ�HNO3�ֽ⣬SO2�ݳ����¶ȹ��ͣ���Ӧ����̫����
(2)
�ٸ���������ԭ����ת����ƽ�÷���ʽ2KMnO4+5NOSO4H+2H2O�TK2SO4+2MnSO4+5HNO3+2H2SO4��
�ʴ�Ϊ2��5��2��H2O��1��2��5��2��
�ڸ��ݵζ��Լ��ļ���˳���֪�ζ��յ�ʱNa2C2O4ǡ�ý�KMnO4����Һ��Ӧ�꣬��ζ��յ������Ϊ�������һ��Na2C2O4����Һ����Һ��dz��ɫ��Ϊ��ɫ�Ұ��������ɫ���ָ���
�ʴ�Ϊ���һ��Na2C2O4����Һ����Һ��dz��ɫ��Ϊ��ɫ�Ұ��������ɫ���ָ���
�۸�����Ŀ���ݣ��ζ�������Na2C2O4����Һ�ĺ���Ϊ31.02mL-1.02mL=30mL�����ݵ���ת���غ��й�ϵʽ��2KMnO4��5Na2C2O4����n��KMnO4��= ![]() ��30mL��0.5000molL-1��10-3L/mL=0.006mol���������������ᣨNOSO4H����Ӧ��KMnO4��0.1L��0.1000molL-1-0.006mol=0.004mol���ɢٵù�ϵʽ��2KMnO4��5NOSO4H��n��NOSO4H��=
��30mL��0.5000molL-1��10-3L/mL=0.006mol���������������ᣨNOSO4H����Ӧ��KMnO4��0.1L��0.1000molL-1-0.006mol=0.004mol���ɢٵù�ϵʽ��2KMnO4��5NOSO4H��n��NOSO4H��= ![]() ��0.004mol=0.01mol�����������ᣨNOSO4H����Ʒ�Ĵ���Ϊ
��0.004mol=0.01mol�����������ᣨNOSO4H����Ʒ�Ĵ���Ϊ![]() ��84.7%��
��84.7%��
�ʴ�Ϊ84.7%��