��Ŀ����
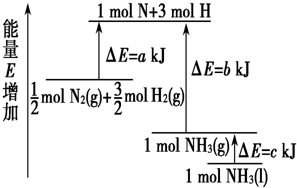
��ѧ��ӦN2+3H2=2NH3�������仯����13ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��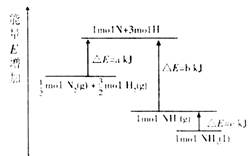
| A��N2(g)+3H2(g)=2NH3(1); ��H=2(a-b-c)kJ��mol-1 |
| B��N2(g)+3H2(g)=2NH3(g); ��H=2(b-a)kJ��mol-1 |
C��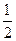 N2(g)+ N2(g)+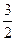 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 H2(g)=NH3(1); ��H=(b+c-a)kJ��mol-1 |
D��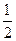 N2(g)+ N2(g)+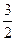 H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 H2(g)=NH3(g); ��H=(a+b)kJ��mol-1 |
A��
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
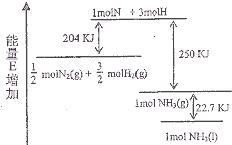 ��ѧ��ӦN2+3H2��2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��ѧ��ӦN2+3H2��2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������| A��N2��g��+H2��g����NH3��1��-46 kJ | B��N2��g��+H2��g����NH3��g��-454 kJ | C��N2��g��+3 H2��g����2 NH3��g��+92 kJ | D��N2��g��+3 H2��g����2 NH3��1��+431.3 kJ |
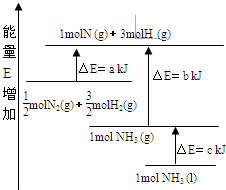 ��ѧ��ӦN2+3H2?2NH3�������仯��ͼ��ʾ��E����ֵ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��ѧ��ӦN2+3H2?2NH3�������仯��ͼ��ʾ��E����ֵ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������| A��N2��g��+3H2��g��?2NH3��l������H=2��a-b-c��kJ?mol-1 | ||||
| B��N2��g��+3H2��g��?2NH3��g������H=2��b-a��kJ?mol-1 | ||||
C��
| ||||
D��
|
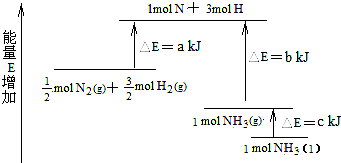 ��2008?���죩��ѧ��ӦN2+3H2=2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������
��2008?���죩��ѧ��ӦN2+3H2=2NH3�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�������