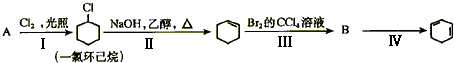
��Ŀ����
��1����ijȲ��B��H2��ּӳɺ�����2,5-�������飬��B�Ľṹ��ʽΪ ��
(2)��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹����У� ����
(3)ijһԪ����A����̼����������Ϊ50.0%���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ������A�Ľṹ��ʽ
��4�� ��һ����������ƿ��ʢ10g11.6%��ijȩ��Һ��Ȼ����������������Һ��ֻ�Ϸ�����ˮԡ�м��ȣ���ȫ��Ӧ��ȥƿ��Һ�壬��ϸϴ������ɺ���ƿ��������4.32g��ͨ�����㣬д������ȩ�Ľṹ��ʽ
��5����0.2 molij�л����0.4 mol O2���ܱ�������ȼ�պ���ΪCO2��CO��H2O�����ᆳ��ŨH2SO4��ŨH2SO4����10.8 g��ͨ�����ȵ�CuO��ַ�Ӧ��CuOʧ��3.2 g�����ͨ����ʯ�ң���ʯ������17.6 g�����������л�����9.2 g ��ǡ�÷�Ӧ�����㲢�ش𣺸��л��������
(1) (CH3)2CHC![]() CCH(CH3)2
CCH(CH3)2
(2) 4
(3) CH2=CHCOOH
��4��![]()
��5���Ҷ���

 ��У����ϵ�д�
��У����ϵ�д�Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
(1)�� �Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��
�� �ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣
д�����з�Ӧ�Ļ�ѧ����ʽ________��
(2)�罫CO2��H2��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ��������_______(�����)��
| A������ | B��ϩ�� | C��Ȳ�� | D������ͬϵ�� |
 CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)��H2O(g) ��H����49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ӷ�Ӧ��ʼ��ƽ�⣬v(H2)��______��������ת���ʣ�_______����ʹƽ����ϵ��n(CH3OH)����Ĵ�ʩ��______��
(3)�罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��
CH4 (g) + 2O2(g)
 CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/molH2(g) + 1/2O2(g)
 H2O(l) ��H2����285.8 kJ/mol
H2O(l) ��H2����285.8 kJ/mol��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
(4)ijͬѧ���������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20����������±���
| �ܽ��(S)/g | �ܶȻ�(Ksp) | ||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
����CO2����ʵ��Լ���_________[�Ca(OH)2����Ba(OH)2��]��Һ��ʵ��ʱ����Ҫ�ⶨ��ҵ���������(����ɱ�״��)�⣬����Ҫ�ⶨ__________��
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
(1)�� �Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��
�� �ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣
д�����з�Ӧ�Ļ�ѧ����ʽ________��
(2)�罫CO2��H2 ��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ��������_______(�����)��
A������ B��ϩ�� C��Ȳ�� D������ͬϵ��
�� �ʵ������ºϳ�ȼ�ϼ״���ˮ�������Ϊ2L���ܱ������У�����2 mol CO2��6 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)
��H����49.0 kJ/mol��
CH3OH(g)��H2O(g)
��H����49.0 kJ/mol��
���CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
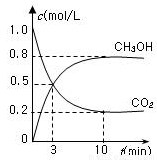
�ӷ�Ӧ��ʼ��ƽ�⣬v(H2)��______��������ת���ʣ�_______����ʹƽ����ϵ��n(CH3OH)����Ĵ�ʩ��______��
(3)�罫CO2��H2 ��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��
CH4 (g) + 2O2(g)  CO2(g)+
2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+
2H2O(l) ��H1���D 890.3 kJ/mol
H2(g) + 1/2O2(g)  H2O(l)
��H2����285.8 kJ/mol
H2O(l)
��H2����285.8 kJ/mol
��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
(4)ijͬѧ���������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20����������±���
|
�ܽ��(S)/g |
�ܶȻ�(Ksp) |
||
|
Ca(OH)2 |
Ba(OH)2 |
CaCO3 |
BaCO3 |
|
0.16 |
3.89 |
2.9��10-9 |
2.6��10-9 |
(˵����KspԽС����ʾ��������ˮ��Һ��Խ�׳���)
����CO2����ʵ��Լ���_________[�Ca(OH)2����Ba(OH)2��]��Һ��ʵ��ʱ����Ҫ�ⶨ��ҵ���������(����ɱ�״��)�⣬����Ҫ�ⶨ__________��
 ��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���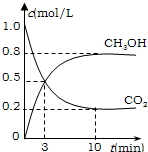 Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���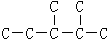 ����������һ�������
����������һ�������