��Ŀ����
 Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о�����1���ٰѺ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ�����ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���壮д�����з�Ӧ�Ļ�ѧ����ʽ
��2���罫CO2��H2 ��1��3������Ȼ�ϣ�
���ʵ������ºϳ�ij����ˮ������������
A������ B��ϩ�� C��Ȳ�� D������ͬϵ��
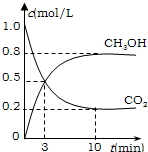
���ʵ������ºϳ�ȼ�ϼ״���ˮ�������Ϊ2L���ܱ������У�����2mol CO2��6mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��g����CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬�����ķ�Ӧ����v��H2��=
��3���罫CO2��H2 ��1��4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H1=-890.3kJ/mol
H2��g��+
| 1 |
| 2 |
��4�������ѧ���������ɫ���ɡ����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״����״�������ȼ�ϵ�أ�д������������Ϊ����ʵļ״�ȼ�ϵ�ظ�����Ӧʽ
��5��ijͬѧ�ó������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20����������±���
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
��2���ٸ���ԭ���غ��жϸ��������ʽ��
����ͼ��֪��10min��ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.8mol/L������v=
| ��c |
| ��t |
����CH3OHƽ��Ũ�ȣ�����n=cV�����n��CH3OH�����ٸ��ݷ���ʽ�����n��H2��������ת���ʵ��ڼ���������ת���ʣ�
�ı����ʹƽ��������Ӧ�ƶ�����������CH3OH�����ʵ������ݴ˽�Ϸ���ʽ�������
��3�����ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ���Ժ��ʵ�ϵ�����мӼ�����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�ļ��㣻
��4���״�������ȼ�ϵ�أ�д������������Ϊ����ʵļ״�ȼ�ϵ�ظ�����Ӧʽ��
��5��̼�����̼�ᱵ���ܶȻ��γɲ����ó������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������ն�����̼����ˮ���ܽ��Ӧ�ýϴ����ܽ��Լ�С���ᵼ�¶�����̼���ղ���ȫ��ͨ���������ⶨ�����ж�����̼�ĺ�����������̼ת��Ϊ̼�ᱵ����������̼�ᱵ����������ȷ��������̼�������
| ||
�ʴ�Ϊ��2KHCO3
| ||
��2������CO2��H2 �����ʵ����ֱ�Ϊ1mol��3mol������Oԭ���غ��֪����H2OΪ2mol�������ɵ�����Cԭ����Hԭ�ӵ����ʵ���֮��Ϊ1mol����3mol��2-2mol��2��=1��2���ʸ��������ʽΪCH2������ϩ����ͨʽ��
�ʴ�Ϊ��B��
����ͼ��֪��10min��ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.8mol/L��v��CH3OH��=
| 0.8mol/L |
| 10min |
ƽ��ʱ��n��CH3OH��=0.8mol/L��2L=1.6mol�����ݷ���ʽ��֪��n��H2��=3��n��CH3OH��=3��1.6mol=4.8mol������������ת����Ϊ
| 4.8mol |
| 6mol |
�÷�Ӧ����Ӧ�������С�ķ��ȷ�Ӧ���ʽ����¶Ȼ��ѹ������H2�����ȣ�������ƽ��������Ӧ�ƶ�������CH3OH�����ʵ�����
�ʴ�Ϊ��0.24mol/��L?min����80%�������¶ȣ����ѹ������H2�����ȣ���
��3����֪����CH4 ��g��+2O2��g���TCO2��g��+2H2O��l����H1=-890.3kJ/mol
��H2��g��+
| 1 |
| 2 |
�ɸ�˹���ɣ��ڡ�4-�ٵ�CO2��g��+4H2��g���TCH4 ��g��+2H2O��l����H=-252.9 kJ/mol
�ʴ�Ϊ��CO2��g��+4H2��g���TCH4 ��g��+2H2O��l����H=-252.9 kJ/mol��
��4���״�ȼ�ϼ��Ե���У��״��ڸ����Ϸ���������Ӧ���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ���缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O���μӷ�Ӧ�������������6.72L����״���£����ʵ���Ϊ0.3mol��O2+2H2O+4e-=4OH-��ת�Ƶ������ʵ���Ϊ1.2mol��
�ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O�� 1.2��
��5��̼�����̼�ᱵ���ܶȻ��γɲ����ó������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������ն�����̼����ˮ���ܽ��Ӧ�ýϴ����ܽ��Լ�С���ᵼ�¶�����̼���ղ���ȫ����ѡ�������������ն�����̼��
ͨ���������ⶨ�����ж�����̼�ĺ�����������̼ת��Ϊ̼�ᱵ����������̼�ᱵ����������ȷ��������̼�������ʵ��ʱ����Ҫ�ⶨ��ҵ����������⣬����Ҫ��̼�ᱵ��������
�ʴ�Ϊ��Ba��OH��2��BaCO3��������

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�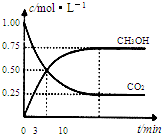 Ϊ��СCO2�Ի�����Ӱ�죬�ڳ�������̼����ͬʱ�������ǿ��CO2�������õ��о�����֪��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol��T1��ʱ�������Ϊ1L�ĺ����ܱ������г���1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ����ƽ��ʱ��ϵѹǿΪP1�����������в���ȷ���ǣ�������
Ϊ��СCO2�Ի�����Ӱ�죬�ڳ�������̼����ͬʱ�������ǿ��CO2�������õ��о�����֪��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol��T1��ʱ�������Ϊ1L�ĺ����ܱ������г���1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ����ƽ��ʱ��ϵѹǿΪP1�����������в���ȷ���ǣ�������| A��0��3min�ڣ�v��CO2����=v ��CH3OH���� | B�������������䣬����ƽ������ϵ�г���1mol��������ϵѹǿ����ƽ�⽫���������ƶ� | C����T1��ʱ������ʼʱ�������г���2molCO2��6mol H2�����ƽ��ʱ������ѹǿΪP2���� P2��2P1 | D��T2��ʱ��������Ӧƽ�ⳣ��Ϊ4.2����T2��T1 |
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
(1)�� �Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��
�� �ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣
д�����з�Ӧ�Ļ�ѧ����ʽ________��
(2)�罫CO2��H2��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ��������_______(�����)��
| A������ | B��ϩ�� | C��Ȳ�� | D������ͬϵ�� |
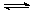 CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)��H2O(g) ��H����49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ӷ�Ӧ��ʼ��ƽ�⣬v(H2)��______��������ת���ʣ�_______����ʹƽ����ϵ��n(CH3OH)����Ĵ�ʩ��______��
(3)�罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��
CH4 (g) + 2O2(g)
 CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/molH2(g) + 1/2O2(g)
 H2O(l) ��H2����285.8 kJ/mol
H2O(l) ��H2����285.8 kJ/mol��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
(4)ijͬѧ���������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20����������±���
| �ܽ��(S)/g | �ܶȻ�(Ksp) | ||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
����CO2����ʵ��Լ���_________[�Ca(OH)2����Ba(OH)2��]��Һ��ʵ��ʱ����Ҫ�ⶨ��ҵ���������(����ɱ�״��)�⣬����Ҫ�ⶨ__________��
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
(1)�� �Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��
�� �ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣
д�����з�Ӧ�Ļ�ѧ����ʽ________��
(2)�罫CO2��H2 ��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ��������_______(�����)��
A������ B��ϩ�� C��Ȳ�� D������ͬϵ��
�� �ʵ������ºϳ�ȼ�ϼ״���ˮ�������Ϊ2L���ܱ������У�����2 mol CO2��6 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)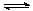 CH3OH(g)��H2O(g)
��H����49.0 kJ/mol��
CH3OH(g)��H2O(g)
��H����49.0 kJ/mol��
���CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
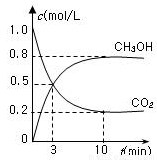
�ӷ�Ӧ��ʼ��ƽ�⣬v(H2)��______��������ת���ʣ�_______����ʹƽ����ϵ��n(CH3OH)����Ĵ�ʩ��______��
(3)�罫CO2��H2 ��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��
CH4 (g) + 2O2(g)  CO2(g)+
2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+
2H2O(l) ��H1���D 890.3 kJ/mol
H2(g) + 1/2O2(g)  H2O(l)
��H2����285.8 kJ/mol
H2O(l)
��H2����285.8 kJ/mol
��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
(4)ijͬѧ���������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20����������±���
|
�ܽ��(S)/g |
�ܶȻ�(Ksp) |
||
|
Ca(OH)2 |
Ba(OH)2 |
CaCO3 |
BaCO3 |
|
0.16 |
3.89 |
2.9��10-9 |
2.6��10-9 |
(˵����KspԽС����ʾ��������ˮ��Һ��Խ�׳���)
����CO2����ʵ��Լ���_________[�Ca(OH)2����Ba(OH)2��]��Һ��ʵ��ʱ����Ҫ�ⶨ��ҵ���������(����ɱ�״��)�⣬����Ҫ�ⶨ__________��
 ��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���