��Ŀ����
Na2CO3��H2O2��ϳɰ�״���壬Na2CO3��xH2O2������������൱��ˮ�ϡ�ע�⣺ʹ�ø�Ũ��H2O2ʱһ��ҪС�ģ���ֹ��ը����ˮ������������������Ư����O2Դ�����ֳ�ȡһ��������Na2CO3��xH2O2������ȡ�ʵ�������¶Ⱥ������������ʾ����ͼ��
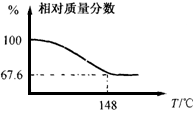
��1���ð�״����Ļ�ѧʽNa2CO3��xH2O2��x=_____________��
��2�����ȹ����У���141��ʱ����Ϊ���ȣ���ԭ������ǣ�_______________
a. Na2CO3��xH2O2�ֽ����
b. ������H2O2�ֽ����
c. Na2CO3��xH2O2�ֽ���������С�ڲ�����H2O2�ֽ�ų�������
��3����ʵ�ϣ�Na2CO3��H2O2��x��1ʱ����Na2CO4��H2O��Na2CO4��������̼���ƣ���ϴ�·��м���������Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ��______________________��
��4��д��Na2CO4��Һ��ϡ���ᷴӦ�����ӷ���ʽ__________________��
��5���������ʲ���ʹ��̼����ʧЧ����_____________
A�� MnO2 B��H2S C��CH3COOH D��NaHCO3
��6��Na2O2��K2O2��CaO2�Լ�BaO2���������������ɹ������⣬Ŀǰʵ������ȡ���������ˮ��Һ��ͨ������ij�ֹ�������������ϡ�������ã����ʺϵĹ���������___________��ԭ����_______________��Ҫʹ��õĹ����������ˮ��Һ�з����������ȡ�Ĵ�ʩ��___________________ ��
��7��д����������ĵȵ�����____________________��һ�����֣�
��2�����ȹ����У���141��ʱ����Ϊ���ȣ���ԭ������ǣ�_______________
a. Na2CO3��xH2O2�ֽ����
b. ������H2O2�ֽ����
c. Na2CO3��xH2O2�ֽ���������С�ڲ�����H2O2�ֽ�ų�������
��3����ʵ�ϣ�Na2CO3��H2O2��x��1ʱ����Na2CO4��H2O��Na2CO4��������̼���ƣ���ϴ�·��м���������Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ��______________________��
��4��д��Na2CO4��Һ��ϡ���ᷴӦ�����ӷ���ʽ__________________��
��5���������ʲ���ʹ��̼����ʧЧ����_____________
A�� MnO2 B��H2S C��CH3COOH D��NaHCO3
��6��Na2O2��K2O2��CaO2�Լ�BaO2���������������ɹ������⣬Ŀǰʵ������ȡ���������ˮ��Һ��ͨ������ij�ֹ�������������ϡ�������ã����ʺϵĹ���������___________��ԭ����_______________��Ҫʹ��õĹ����������ˮ��Һ�з����������ȡ�Ĵ�ʩ��___________________ ��
��7��д����������ĵȵ�����____________________��һ�����֣�
��1��x��1.5
��2��c
��3��2Na2CO4��2Na2CO3��O2��
��4��2CO42����4H����2CO2����O2����H2O
��5��D
��6��BaO2�����ɹ�����������ᱵ��������ȥ�������ý�Ϊ�����Ĺ�������ˮ��Һ����ѹ����
��7��F2��Br2��I2��ClO-��BrO-��IO- ������һ�����ּ��ɣ�
��2��c
��3��2Na2CO4��2Na2CO3��O2��
��4��2CO42����4H����2CO2����O2����H2O
��5��D
��6��BaO2�����ɹ�����������ᱵ��������ȥ�������ý�Ϊ�����Ĺ�������ˮ��Һ����ѹ����
��7��F2��Br2��I2��ClO-��BrO-��IO- ������һ�����ּ��ɣ�

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ
����������ȷ���ǣ�������
| A����Na2O2��H2O��Ӧ�У�Na2O2����������H2O�ǻ�ԭ�� | B��Na2O��Na2O2�������Ӹ����������Ӹ���֮�Ⱦ�Ϊ1��2 | C�����Ȼ�����Һ����NaHCO3��Na2CO3��Һ | D��������ȵ�Na2CO3��NaHCO3�ֱ����������ᷴӦ���������ɵ�CO2һ���� |






