��Ŀ����
��16�֣���ij��ȤС����ʵ�����ü����Ҵ���ŨH2SO4���廯�ƺ�����ˮ�Ļ�������Ʊ������飬���鷴Ӧ�IJ��ָ������̽������������ʡ�
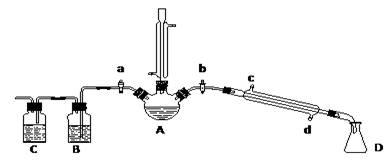
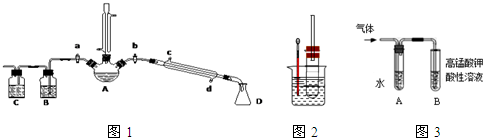
��һ����������Ʊ�������ļ��飺���������ͼװ�ã����мг�������������������ȴˮ��û�л����������ʵ�鲽�裬�ش��������⣺
��1������A��������
��2���Ʊ������У������������ˮ����Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��3������Ƭ�̺�A�еĻ������ֳȺ�ɫ���óȺ�ɫ���ʿ�����_____ _______
��4�������ϣ�������Ӧ�ĸ����ﻹ�����У����ѣ�CH3CH2��O��CH2CH3������ϩ���廯���
�� ���鸱�������Ƿ����廯�⣺Ϩ��ƾ��ƣ�����ֱ�������Ϸ��������ӡ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��顣B��C��Ӧʢ�ŵ��Լ��ֱ��� ��
�� ���鸱�������Ƿ������ѣ�ͨ����������Ǽ������ò����к��С���CH2CH3�����ţ���ȷ���������д������ѡ�����Ը�ͬѧ�Ĺ۵�������ۣ�
��5������ȥ�������е���������Br2���������������ʺϵ��� ��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
���������������ʵ�̽����
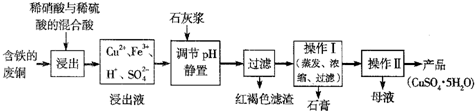
����ͼʵ��װ��(����̨���ƾ�����) ��֤����������ʣ�

�����Թ��м���10 mL6mol/L NaOH��Һ��2 mL �����飬��
II�����Թ���ͼ�̶���ˮԡ���ȡ�
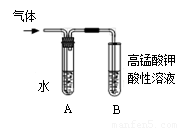
(1)�۲쵽______ _____����ʱ��������������NaOH��Һ����ȫ��Ӧ��
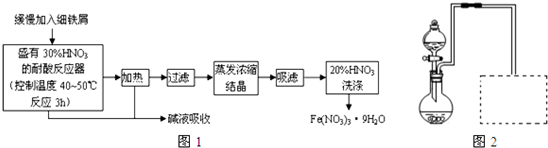
(2)Ϊ֤����������NaOH�Ҵ���Һ�з���������ȥ��Ӧ�������ɵ�����ͨ����ͼװ�á�A�Թ��е�ˮ�������� ������A�Թܣ�B�Թ��е��Լ�ӦΪ ��
(16��) ��16�֣�
��һ��
��1��������ƿ��2�֣���2�� abc ��2�֣� ��3��Br2��2�֣�
��4���� ���������Ȼ�̼���л��ܼ�����1�֣� �� ��������ʯ����Һ�����һ�ּ��ɣ���1�֣�
�� ����ȷ��1�֣� ��������Ҳ���һ���1�֣�
��5�� c ��2�֣�
������
(1)Һ�岻�ֲ㣨2�֣���(2) �����Ҵ� ��1�֣� �� ��ˮ ��1�֣�
��������
�����������1��������ƿ��
��2����Ӧ�м���������ˮ����ֹ��Ӧ����ʱ������������ĭ�����ٸ��������ѵ����ɺͱ���HBr �Ļӷ�����ѡabc��
��3����Ϊ�Ⱥ�ɫҺ�壬�ʴ�����Ϊ�塣
��4���ټ����廯�⣬������������Һ��ʯ����Һ���飬����Ҫ��ȥ���е��л�����ԣ��������ͨ���л��ܼ�����ͨ����������Һ��ʯ����Һ�����������Ȼ�̼���л��ܼ�������������ʯ����Һ��
��������������Ҳ���һ����ʴ�ͬѧ���жϴ���
��5����ȥ�������е���������Br2����NaI������ⵥ�����ʣ�a�����������ƻ�����������ˮ�⣬b��������������ֻ���巴Ӧ���������鷴Ӧ��c��ȷ�����Ȼ��ض�����Ӧ��d����
������
��1����Ϊ������������������Һ�����ܣ�����ֲַ㣬��Һ�岻�ֲ�ʱ��˵�������鷴Ӧ����ȫ��
��2����������NaOH�Ҵ���Һ�з�Ӧ�����ɵ���������Ҵ����������Խ����������ͨ��ˮ�ɳ�ȥ�Ҵ�������A�Թܣ�B�Թ���װ���������Һ����������е��Ҵ�Ҳ����ʹ���������ɫ���ʸ������Ҫ��Ϊ��ˮ���Ҵ�������ˮ��Ӧ��
���㣺���������ȡ�����ʡ�
��������Ϥ���������ȡ��±���������ʡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�