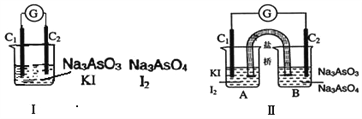
��Ŀ����
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺
��ѡ�Լ���Na2SO4��Һ��K2CO3��Һ��K2SO4��Һ�����ᡣ
��1���Լ�a��_________�������Լ�b��������Ӧ�����ӷ���ʽΪ_________��
��2���÷����ܷ�ﵽʵ��Ŀ��_____(��ܡ����ܡ�)�������ܣ�Ӧ��θĽ�?_____�����ܣ����ʲ��ûش�)��
��3����Ҫ�ⶨԭ�������BaCl2����������������Ҫȷ�������������������ٻ�Ҫ��õ�������____��������
��4��������3�����Ʊ���KCl��������0.1mol/LKCl��Һ450mL���ش��������⣺
��i�����ƹ�������Ҫ�õ��IJ����������ձ�������������Ͳ����ͷ�ιܡ�____________��
��ii����Ҫ��������ƽ����______________gKCl���塣
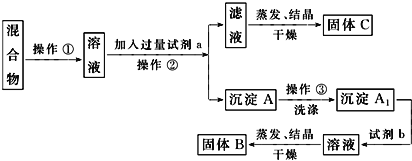
���𰸡�K2CO3��Һ BaCO3+2H+��Ba2++CO2��+H2O ���� Ӧ�ڲ����ڵ���Һ�м������������������ᾧ ����A1(�����B) 500mL����ƿ 3.7
��������
����KCl��BaCl2���ֹ��������������ˮ��Ȼ��������K2CO3ʹBaCl2ת��Ϊ���������˺�����������������BaCl2��Һ�����������ᾧ�������ɵù���BaCl2��������������ҺΪKCl��K2CO3�Ļ��������ᾧ�õ�����CΪKCl��K2CO3������AΪBaCO3��ϴ�Ӻ����ᣬ�����õ�����BΪBaCl2���Դ������
��1�������Լ�a����BaCl2��Ӧ����BaCO3����������Լ�a��K2CO3��Һ��̼�ᱵ����ϴ�Ӹ��������Լ�bת��Ϊ�Ȼ�����Һ�����Լ�b�����ᣬ��Ӧ�����ӷ���ʽΪBaCO3+2H+��Ba2++CO2��+H2O��
��2���������ڵõ�����Һ�к��й���K2CO3��ֱ�������õ����Ȼ����л���̼��أ�Ӧ�ȼ��������ữ��Ȼ���������ᾧ��
��3���ⶨ�Ȼ����������������ɼ���̼�ᱵ�������Ȼ�����������������Ҫȷ�������������������ٻ�Ҫ��õ������dz���A1�����B��������
��4��������3�����Ʊ���KCl��������0.1mol/LKCl��Һ450mL������û��450mL����ƿ������Ҫ500mL����ƿ�������ƹ�������Ҫ�õ��IJ����������ձ�������������Ͳ����ͷ�ιܺ�500mL����ƿ�������ҪKCl�����������0.5L��0.1mol/L��74.5g/mol��3.725g������������ƽֻ�ܶ�����0.1g������Ҫ��������ƽ������������3.7g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�