题目内容
【题目】(1) 基态Si原子中,电子占据的最高能层符号为________,该能层具有的原子轨道数为_________。
(2) CH3COOH中C原子轨道杂化类型为_________;1molCH3COOH分子含有σ键的数目为_________。
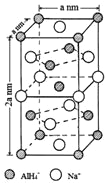
(3) 复化硼(BN)晶体有多种相结构。六方相氮化硼是通常存在的稳定相,与石墨相似,具有层状结构,可作高温润滑剂。立方相氮化硼是超硬材料,有优异的耐磨性。它们的晶体结构如图所示。

①基态硼原子的电子排布式为__________。
②六方相氮化硼晶体层内一个硼原子与相邻氮原子构成的空间构型为__________,分析其结构,与石墨相似却不导电,原因是__________。
③NH4BF4 (氟硼酸铵)是合成氮化硼纳米管原料之一。1mol NH4BF4 含有__________mol 配位键。
(4) Ge单晶具有金刚石型结构,其中Ge原子的杂化方式为_______,微粒之间存在的作用力是__________。
(5) 晶胞有两个基本要素:
①原子坐标参数,表示晶胞内部各原子的相对位置,下图为金刚石的晶胞,其中原子坐标参数A为(0,0 ,0);B为(![]() ,0,
,0, ![]() );C为(
);C为(![]() ,
,![]() ,0)。则D原子的坐标参数为__________。
,0)。则D原子的坐标参数为__________。
②晶胞参数,描述晶胞的大小和形状,已知金刚石晶胞参数acm,其密度为__________g·cm-3(列出计算式即可)。
【答案】 M 9 sp3和sp2 7NA(或7×6.02×1023) 1s22s2p1 平面三角形 层状结构中没有自由移动的电子 2 sp3 共价键 (![]() ;
; ![]() ;
; ![]() )
) ![]()
【解析】(1)Si原子核外电子数为14,基态原子核外电子排布为1s22s22p63s23p2,电子占据的最高能层符号为M,该能层具有的原子轨道数为1+3+5=9;故答案为:M;9;
(2)CH3COOH中C原子分别形成4个、3个δ键,没有孤对电子,分别为sp3杂化、sp2杂化,CH3CHOOH分子中含有1个C-C、3个C-H、1个C-O、1个C=O、1个O-H等化学键,则1mol CH3COOH分子中含有σ键的数目为7mol或7×6.02×1023,故答案为:sp3和sp2;7mol或7×6.02×1023;
(3)①基态硼原子核外有5个电子,分别位于1s、2s、2p能级,根据构造原理知其基态的电子排布式1s22s22p1,故答案为:1s22s22p1;
②六方相氮化硼晶体层内一个硼原子与相邻氮原子形成3个共价单键,且B原子不存在孤电子对,所以构成的空间构型为平面三角形,该物质的层状结构中不存在自由移动的电子,所以不导电,故答案为:平面三角形;层状结构中没有自由移动的电子;
③一个NH4BF4中N原子和其中一个H原子之间存在配位键、B原子和其中一个F原子之间存在一个配位键,所以含有2个配位键,则1mol NH4BF4含有2mol配位键,故答案为:2;
(4)Ge单晶具有金刚石型结构,Ge原子与周围4个Ge原子形成正四面体结构,向空间延伸的立体网状结构,属于原子晶体,Ge原子之间形成共价键,Ge原子杂化轨道数目为4,采取sp3杂化,故答案为:sp3;共价键;
(5)①D与周围4个碳原子形成正四面体结构,D与顶点A的连线处于晶胞体对角线上,过面心B、C及上底面面心原子的平面且平行侧面将晶胞2等分,同理过D原子的且平行侧面的平面将半个晶胞再2等份,可知D处于到各个面的![]() 处,则D原子的坐标参数为(
处,则D原子的坐标参数为(![]() ,
, ![]() ,
, ![]() ),故答案为:(
),故答案为:(![]() ,
, ![]() ,
, ![]() );
);
②晶胞中C原子数目为4+8×![]() +6×
+6×![]() =8,结合阿伏伽德罗常数,可知出晶胞的质量为
=8,结合阿伏伽德罗常数,可知出晶胞的质量为![]() g,晶胞参数acm,其密度为
g,晶胞参数acm,其密度为![]() g÷(acm)3=
g÷(acm)3=![]() g·cm-3,故答案为:
g·cm-3,故答案为: ![]() 。
。

【题目】(1)一定温度下,Ksp[Mg3(PO4)2]=6.0×10-29,Ksp[Ca3(PO4)2]=6.0×10-26。向浓度均为 0.20mol·L-1的 MgCl2 和 CaCl2 混合溶液中逐滴加入 Na3PO4,先生成沉淀________(填化学式);当测得溶液其中一种金属阳离子沉淀完全(浓度小于10-5mol·L-1)时,溶液中的另一种金属阳离子的物质的量浓度c________。
(2)毒重石的主要成分BaCO3(含Ca2+、Mg2+、Fe3+等杂质),实验室利用毒重石制备BaCl2·2H2O的流程如下:

已知: Ksp(BaC2O4)=1.6×10-7, Ksp(CaC2O4)=2.3×10-9
Ca2+ | Mg2+ | Fe3+ | |
开始沉淀时的pH | 11.9 | 9.1 | 1.9 |
完全沉淀时的pH | 13.9 | 11.1 | 3.7 |
①毒重石用盐酸浸取前需充分研磨,目的是________。
②加入NH3·H2O调节pH=8 可除去________(填离子符号),滤渣Ⅱ中含________(填化学式)。加入H2C2O4时应避免过量,原因是________________。