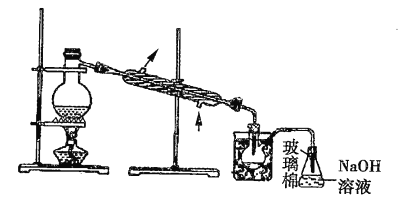
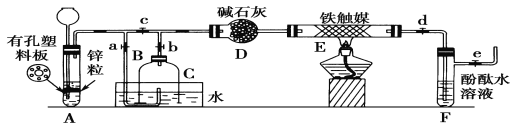
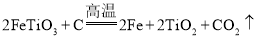��Ŀ����
����Ŀ��(1)��������������������O3����NO���ˮϴ���ɲ���HNO3��O2���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
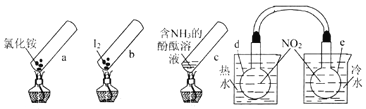
(2)��ͼ����NH3�ѳ�������NO��ԭ����
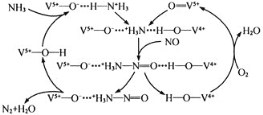
�ٸ�����ԭ���У�NO����ת��Ϊ__________(�ѧʽ)��H2O��
�ڵ�����2mLNH3��0.5molO2ʱ����ȥ��NO�ڱ�״���µ����Ϊ________L��
(3)�ü�Һ������Ŀǰ�о��Ŀ���֮һ��
�ٽ�NO��NO2ͨ��ʯ�����п��Ʊ���Ҫ�Ĺ�ҵԭ��Ca(NO3)2���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO2)��n(NO)>1��1����ᵼ��_____________����n(NO2):n(NO)<1��1����ᵼ��_____________��
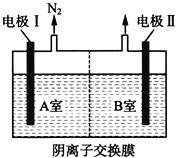
�ڽ�����������Һ�����õ���NaNO2��NaNO3�Ļ��Һ��NaOH��Һ�ֱ�ӵ���ͼ��ʾ�ĵ����н��е�⡣д��A��NO2-�����ĵ缫��Ӧʽ��______________��
���𰸡�3O3+2NO+H2O=2HNO3+3O2 N2 44.8 ��ƷCa(NO2)2��Ca(NO3)2�������� �ų���������NO�������� 2NO2-+6e-+4H2O=8OH-+N2��
��������
(1)O3����NO���ˮϴ�ɲ���HNO3��O2�����ԭ���غ�͵�ʧ�����غ�д����Ӧ����ʽ��
(2)����ͼ��֪��Ӧ��Ϊ������һ�������Ͱ�������������Ϊ������ˮ��
�ڸ��ݰ���ʧȥ�ĵ��ӵ����ʵ�������NO�������õ��ĵ��������ʵ������㣻
(4)����n(NO2)��n(NO)<1��1����һ��������������n(NO2)��n(NO)>1��1�����������������
��ͨ��A�Ҳ�����N2����֪A���ĵ������ҺΪNaNO3��NaNO2�Ļ����Һ��NO2-��A���ŵ�ΪN2����AΪ��������B��Ϊ�������������ҺΪNaOH��Һ��OH-��B���ŵ磬�ݴ˷�����
(1)O3����NO���ˮϴ�ɲ���HNO3��O2�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ����ʽΪ��3O3+2NO+H2O=2HNO3+3O2��
(2)����ͼ��֪��Ӧ��Ϊ������һ�������Ͱ�������������Ϊ������ˮ������NO����ת��ΪN2��H2O��
��������һ�������Ͱ�����Ӧ���ɵ�����ˮ����Ӧ�а���ʧȥ�ĵ��ӵ����ʵ�������NO�������õ��ĵ��������ʵ�����2mol NH3ת��ΪN2ʧȥ6mol���ӣ�0.5mol O2�õ�2mol���ӣ���NOת��ΪN2�õ��ĵ���Ϊ4mol������NO�����ʵ���Ϊ2mol�����ڱ�״���µ����V(NO)=2mol��22.4L/mol=44.8L��
(3)����n(NO)��n(NO2)<1��1����NO2������������������ʯ���鷴Ӧ����Ca(NO3)2�����²�ƷCa(NO2)2��Ca(NO3)2�������ߣ���n(NO)��n(NO2)>1��1����NO�������ŷ�������NO�������ߣ�
��NO2-�������õ��ӱ���ԭΪN2������A����NO2-�ŵ�ĵ缫��ӦʽΪ��2NO2-+6e-+4H2O=8OH-+N2����

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ����ҵ�����õ�����ࣨ��Ҫ����Fe2O3��CuO��Cr2O3���������������ʣ�����ͭ���Ƚ���������������ͼ��ʾ��

��֪�������ʳ�����pH���������CaSO4Ԫ���ܽ�����ߣ���ͼ����
Fe3�� | Cu2�� | Cr3�� | |
��ʼ���� | 2.1 | 4.7 | 4.3 |
��ȫ���� | 3.2 | 6.7 | 5.6 |

��1���ڽ��������г�������Fe2��SO4��3��Cr2��SO4��3�⣬��Ҫ����_____________���ѧʽ����
��2���ڳ��������У���Ҫ��ȥFe3����CaSO4���������ز������ټ���ʯ�������pH��______��
�ڽ���Һ���ȵ�80����____________��
��3��д����ԭ�����м���NaHSO3����Cu2O��������ӷ���ʽ��_________���˲����м���NaHSO3�õ�Cu2O�IJ���Ϊ95%����NaHSO3�����������˷��Լ��⣬������ֵ�������______________��