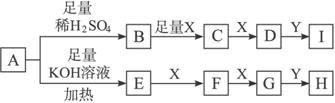
��Ŀ����
��A���ʵĻ�ѧʽΪM��OH��2����������ˮ�Ƴ�ϡ��Һ������Һ�����ԣ�����Һ�д��ڣ�M2++2OH-
 M��OH��2
M��OH��2  2H++MO22-
2H++MO22-�ش�������Ŀһ���á�������С�������䡱���
��1���������������������£�25C° ʱ��������ˮ�м���A���ʺ������ǰ�Ƚϣ�
����ˮ�������C��OH-�� ��C��H+�� ��ˮ�ĵ���� ��Kw
��2��25C° ʱ����A��ϡ��Һ�м����������ռ���壮��ˮ�ĵ���� ����Һ��pH
�������£��ס�����ƿ��ˮ��Ũ�ȷֱ�Ϊ1mol/L��0.1mol/L����ס�����ƿ��ˮ��C��OH-��֮��Ϊ 10 ������ڡ����ڻ�С�ڣ�
���𰸡���������1�����ݵ���ƽ���Ӱ��������������
��2�����ˮ�ĵ������������ã�ˮ�ĵ���̶ȼ�С��
��3��������ƿ��ˮ��Ũ���Լ��������������������Ũ�ȣ�
����⣺��1������ΪA����Һ�����ԣ�����25C°ʱ��������ˮ�м���A���ʺ�ˮ�ĵ�����Ӱ�죬������ˮ�������C��OH-����C��H+����ˮ�ĵ���ȡ�Kw�����䣬�ʴ�Ϊ�����䣻���䣻���䣻���䣻
��2��25C°ʱ����A������ϡ��Һ�м����������ռ���壬�൱�ڼ�����Һ�����ˮ�ĵ������������ã�ˮ�ĵ���̶ȼ�С���ʴ�Ϊ����С������
��3����ˮ��Ũ��ԽС��������Խ����ѭԽϡԽ�����ԭ����0.1mol/L�İ�ˮ�Ƚ������룬������Ũ�ȴ���1mol/L��ˮ������������Ũ�ȵ�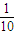 �����������ֵӦ��С��10���ʴ�Ϊ��С�ڣ�
�����������ֵӦ��С��10���ʴ�Ϊ��С�ڣ�
���������⿼��ѧ��������ʵĵ���ƽ���Ӱ�������Լ�����ƽ���Ӧ��֪ʶ�����Ը�����ѧ�������ش��ѶȲ���
��2�����ˮ�ĵ������������ã�ˮ�ĵ���̶ȼ�С��
��3��������ƿ��ˮ��Ũ���Լ��������������������Ũ�ȣ�
����⣺��1������ΪA����Һ�����ԣ�����25C°ʱ��������ˮ�м���A���ʺ�ˮ�ĵ�����Ӱ�죬������ˮ�������C��OH-����C��H+����ˮ�ĵ���ȡ�Kw�����䣬�ʴ�Ϊ�����䣻���䣻���䣻���䣻
��2��25C°ʱ����A������ϡ��Һ�м����������ռ���壬�൱�ڼ�����Һ�����ˮ�ĵ������������ã�ˮ�ĵ���̶ȼ�С���ʴ�Ϊ����С������
��3����ˮ��Ũ��ԽС��������Խ����ѭԽϡԽ�����ԭ����0.1mol/L�İ�ˮ�Ƚ������룬������Ũ�ȴ���1mol/L��ˮ������������Ũ�ȵ�
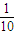 �����������ֵӦ��С��10���ʴ�Ϊ��С�ڣ�
�����������ֵӦ��С��10���ʴ�Ϊ��С�ڣ����������⿼��ѧ��������ʵĵ���ƽ���Ӱ�������Լ�����ƽ���Ӧ��֪ʶ�����Ը�����ѧ�������ش��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 M��OH��2
M��OH��2  2H++MO22-
2H++MO22-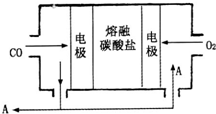 ��2013?����ģ�⣩�Ƽ��仯������й㷺����;��
��2013?����ģ�⣩�Ƽ��仯������й㷺����;��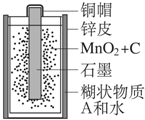 �Ͼɸɵ�������������ǿ��Ի������õģ�ij���ͺŸɵ�ص�������ͼ��ʾ����ش��������⣺
�Ͼɸɵ�������������ǿ��Ի������õģ�ij���ͺŸɵ�ص�������ͼ��ʾ����ش��������⣺