��Ŀ����
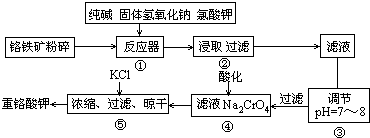
��15�֣��ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3��Ϊԭ������,ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3+24NaOH+7KClO3 12Na2CrO4+3Fe2O3
+7KCl+12H2O��
12Na2CrO4+3Fe2O3
+7KCl+12H2O��

�Իش��������⣺
��1���ڷ�Ӧ�����У���Na2CrO4���ɣ�ͬʱFe2O3ת��ΪNaFeO2������SiO2��Al2O3�봿�Ӧת��Ϊ�������Σ�д����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��
��
��2�������۵�Ŀ����ʲô���ü�Ҫ������˵����
��
��3���������У��ữʱ��CrO42��ת��ΪCr2O72��,д��ƽ��ת�������ӷ���ʽ��
��
��4����ȡ�ظ��������2.5000g���250mL��Һ��ȡ��25.00mL�ڵ���ƿ�У�����10mL 2mol/LH2SO4�������⻯��(���Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���
��I2+2S2O32��=2I��+S4O62����
���жϴﵽ�ζ��յ�������ǣ� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL,�����ò�Ʒ���ظ���صĴ��ȣ��������������������ʲ����뷴Ӧ�� ��������С�������λ����
(ÿ��3�֣���15��)
��1��Al2O3 + Na2CO3 ==2NaAlO2 + CO2
��2�����ڹ����ƺ�ƫ�����Ʒ���ˮ�⣬����pHֵ������ˮ��ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ���Ӷ���ȥSiO32����AlO2��
��3��2CrO42��+2H+ 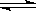 Cr2O72��+H2O
Cr2O72��+H2O
��4���ٵ��μ����һ�������������Һ����Һ��ɫ��ȥ�����ڰ��������ɫ���ٸı�
�� 94.08
��������

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�