��Ŀ����
����Ŀ������ֱ�������Ƽ״��Ǹ�����ս�ԵĿ��⣬Sen����CF3COOHˮ��Һ�гɹ�������ת��ΪCF3COOCH3(ˮ������CH3OH)���䷴Ӧ������ͼ��ʾ������˵����ȷ���ǣ� ��
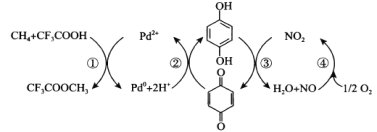
A.������Ӧ���ܷ�ӦʽΪCH4��CF3COOH��![]() O2��CF3COOCH3
O2��CF3COOCH3
B.CF3COOCH3ˮ������CH3OH�ķ�ӦʽΪCF3COOCH3��H2O��CF3COOH��CH3OH
C.Pd2���Ǹ÷�Ӧ���м����
D.ÿ����1molCH3OH�����ı�״����O2�����Ϊ22.4L
���𰸡�B
��������
A�����ݢ٢ڢܼۢӺͿɵ�������Ӧ���ܷ�ӦʽΪCH4��CF3COOH��![]() O2��CF3COOCH3+H2O����Aѡ�����
O2��CF3COOCH3+H2O����Aѡ�����
B��CF3COOCH3����ˮ�ⷴӦ�IJ���ΪCF3COOH��CH3OH��������CH3OH�ķ�ӦʽΪCF3COOCH3��H2O��CF3COOH��CH3OH����Bѡ����ȷ��
C����Ӧ����Pd2�����뷴Ӧ����Ӧ���������ɵ����ʵ�����Pd2������Pd2��Ϊ���������м�����Cѡ�����
D�����ݷ�ӦCH4��CF3COOH��![]() O2��CF3COOCH3+H2O��CF3COOCH3��H2O��CF3COOH��CH3OH�ɵ��Ƽ״��ķ�Ӧ����ʽΪCH4��
O2��CF3COOCH3+H2O��CF3COOCH3��H2O��CF3COOH��CH3OH�ɵ��Ƽ״��ķ�Ӧ����ʽΪCH4��![]() O2��CH3OH����ÿ����1molCH3OH�����ı�״����O2�����Ϊ11.2L����Dѡ�����
O2��CH3OH����ÿ����1molCH3OH�����ı�״����O2�����Ϊ11.2L����Dѡ�����
�ʴ�ѡB��

����Ŀ��ijͬѧ������ͼװ�ý�����Ȫʵ�飬��֪Բ����ƿ�ڳ���X���壬��ͷ�ι���װ������YҺ�壬�ձ���װ������ZҺ�壬��������ܽ�����Ȫʵ��������Һ��һ���ܳ���������ƿ����
X���� | Y�Լ� | Z�Լ� | |
A | NO2 | H2O | H2O |
B | HCl | H2O | H2O |
C | HCl��O2������� | H2O | H2O |
D | NH3��N2������� | H2O | H2O |
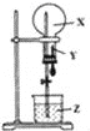
A. A B. B C. C D. D
����Ŀ��ijͬѧ�����в�������500mL 0.200mol��L��1 Na2CO3��Һ����ش��й����⡣
ʵ�鲽�� | �й����� |
(1)��������Na2CO3������ | ��ҪNa2CO3������Ϊ_________g�� |
(2)����Na2CO3���� | ������������Ҫ�õ���������_____ |
(3)��Na2CO3����100mL�ձ��� | Ϊ�ӿ��ܽ����ʣ��ɲ�ȡ�Ĵ�ʩ��___ |
(4)���ձ��е���Һת����500mL����ƿ�� | Ϊ��ֹ��Һ������Ӧ��ȡ�Ĵ�ʩ��____________________________________________________________________________ |
(5)������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱӦע���������______ |
(6)����Ϊ�������������Ƶ�Na2CO3��Һ��Ũ���Ƿ�Ϊ0.200mol��L��1����˵�����ɡ�________


