��Ŀ����
��2013?����һģ��ˮ������֮Դ��Ҳ�ǻ�ѧ��Ӧ�е����ǣ���ش��������⣺
I��ˮ��һ�ֵ���ʣ�����������������������ͬ��������������뷽��ʽΪ
�������෴Ӧ��H2O���ݲ�ͬ�ġ���ɫ����������ѧ֪ʶ��д���йط�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��1��H2O�����û���Ӧ������X+W��Y+V��
��֪X��Y�ֱ��Ƕ���������Ԫ���γɵ����ֵ��ʣ�W��V�ǻ����
��W��ˮ������ԭ�����÷�Ӧ�Ļ�ѧ����Ϊ
��V��ˮ����ѧ����ʽΪ
��2��ˮ��������ԭ��Ӧ�Ȳ���������Ҳ���ǻ�ԭ����
A��B����ѧ��ѧ�����������ɶ�����Ԫ����ɵ���ɫ���壬���Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����д��A��B��ˮ��Ӧ�Ļ�ѧ����ʽ��
��A+H2O
��B+H2O
��3��ij���ʻ�ѧʽΪNH5���������ǹ�̬������ˮ���ҷ�Ӧ�ų��������壮��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�������ж�NH5��ˮ�ķ�Ӧ������ȷ����
A��NH5��ˮ��Ӧʱ��NH5�������� B��NH5��ˮ��Ӧʱ��NH5�������������ǻ�ԭ��
C��NH5��ˮ��Ӧʱ��NH5�ǻ�ԭ�� D��NH5��NH3����ˮ����Һ���ʼ��ԣ�
I��ˮ��һ�ֵ���ʣ�����������������������ͬ��������������뷽��ʽΪ
H2O+H2O?OH-+H3O+
H2O+H2O?OH-+H3O+
���������෴Ӧ��H2O���ݲ�ͬ�ġ���ɫ����������ѧ֪ʶ��д���йط�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��1��H2O�����û���Ӧ������X+W��Y+V��
��֪X��Y�ֱ��Ƕ���������Ԫ���γɵ����ֵ��ʣ�W��V�ǻ����
��W��ˮ������ԭ�����÷�Ӧ�Ļ�ѧ����Ϊ
2F2+2H2O�TO2+4HF
2F2+2H2O�TO2+4HF
����V��ˮ����ѧ����ʽΪ
O2+2H2S�TS+2H2O
O2+2H2S�TS+2H2O
����2��ˮ��������ԭ��Ӧ�Ȳ���������Ҳ���ǻ�ԭ����
A��B����ѧ��ѧ�����������ɶ�����Ԫ����ɵ���ɫ���壬���Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����д��A��B��ˮ��Ӧ�Ļ�ѧ����ʽ��
��A+H2O
Cl2+H2O�THCl+HClO
Cl2+H2O�THCl+HClO
����B+H2O
3NO2+H2O�T2HNO3+NO
3NO2+H2O�T2HNO3+NO
����3��ij���ʻ�ѧʽΪNH5���������ǹ�̬������ˮ���ҷ�Ӧ�ų��������壮��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�������ж�NH5��ˮ�ķ�Ӧ������ȷ����
CD
CD
����ѡ���A��NH5��ˮ��Ӧʱ��NH5�������� B��NH5��ˮ��Ӧʱ��NH5�������������ǻ�ԭ��
C��NH5��ˮ��Ӧʱ��NH5�ǻ�ԭ�� D��NH5��NH3����ˮ����Һ���ʼ��ԣ�
������I��ˮ�����OH-��H3O+���ӣ����ߵ���������ȣ�
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ������ݴ�д����Ӧ����ʽ��
��V��ˮ����WΪ�⻯���XΪO2��
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��3��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�����ԣ�NH5����笠����Ӻ����������γɵ����ӻ�����������Ȼ�什ṹ����ˮ��Ӧ�����������壬NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O������Ԫ�ػ��ϼ��ж��������ͻ�ԭ����
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ������ݴ�д����Ӧ����ʽ��
��V��ˮ����WΪ�⻯���XΪO2��
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��3��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�����ԣ�NH5����笠����Ӻ����������γɵ����ӻ�����������Ȼ�什ṹ����ˮ��Ӧ�����������壬NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O������Ԫ�ػ��ϼ��ж��������ͻ�ԭ����
����⣺I��ˮ�����OH-��H3O+���ӣ����ߵ���������ȣ�����뷽��ʽΪH2O+H2O=OH-+H3O+���ʴ�Ϊ��H2O+H2O=OH-+H3O+��
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ�����������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2F2+2H2O�TO2+4HF��
�ʴ�Ϊ��2F2+2H2O�TO2+4HF��
��V��ˮ����WΪ�⻯���XΪO2�������������û���ӦΪO2+2H2S=2H2O+2S�����ʴ�Ϊ��O2+2H2S=2H2O+2S����
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��Cl2��ˮ��Ӧ����HCl��HClO����ѧ����ʽΪCl2+H2O=HCl+HClO���ʴ�Ϊ��Cl2+H2O=HCl+HClO��
��NO2��ˮ��Ӧ����NO��HNO3����ѧ����ʽΪ3NO2+H2O=2HNO3+NO���ʴ�Ϊ��3NO2+H2O=2HNO3+NO��
��3��NH5��ˮ�����ķ�Ӧ����ʽΪ��NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O����Ӧ�У�NH5�е�HԪ��ʧ���ӣ�H2O�е�HԪ�صõ��ӣ�����NH5�ǻ�ԭ����H2O����������NH5��NH3����ˮ�����ɰ�ˮ��Һ��������Һ���ʼ��ԣ���ѡCD��
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ�����������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2F2+2H2O�TO2+4HF��
�ʴ�Ϊ��2F2+2H2O�TO2+4HF��
��V��ˮ����WΪ�⻯���XΪO2�������������û���ӦΪO2+2H2S=2H2O+2S�����ʴ�Ϊ��O2+2H2S=2H2O+2S����
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��Cl2��ˮ��Ӧ����HCl��HClO����ѧ����ʽΪCl2+H2O=HCl+HClO���ʴ�Ϊ��Cl2+H2O=HCl+HClO��
��NO2��ˮ��Ӧ����NO��HNO3����ѧ����ʽΪ3NO2+H2O=2HNO3+NO���ʴ�Ϊ��3NO2+H2O=2HNO3+NO��
��3��NH5��ˮ�����ķ�Ӧ����ʽΪ��NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O����Ӧ�У�NH5�е�HԪ��ʧ���ӣ�H2O�е�HԪ�صõ��ӣ�����NH5�ǻ�ԭ����H2O����������NH5��NH3����ˮ�����ɰ�ˮ��Һ��������Һ���ʼ��ԣ���ѡCD��
���������⿼��������ԭ��Ӧ�Լ�������ƶϵ�֪ʶ����Ŀ��Ϊ�ۺϣ��Ѷ��еȣ�����ע�ⳣ��Ԫ�ػ�����֪ʶ�Ļ��ۣ��ι̰��ջ���֪ʶ��������Ŀ�ɽ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��2013?����һģ�������й�ʵ�����������ͽ��ͻ���۶���ȷ���ǣ�������
|
 ��2013?����һģ��Ԫ��X�ĵ��ʼ�X��Y�γɵĻ������ܰ���ͼ��ʾ�Ĺ�ϵ����ת��������m��n���Ҿ�Ϊ��������������˵����ȷ���ǣ�������
��2013?����һģ��Ԫ��X�ĵ��ʼ�X��Y�γɵĻ������ܰ���ͼ��ʾ�Ĺ�ϵ����ת��������m��n���Ҿ�Ϊ��������������˵����ȷ���ǣ�������
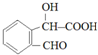
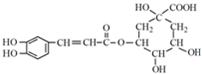 ����һ�ֿ�����ҩ�������ͼת����ϵ��
����һ�ֿ�����ҩ�������ͼת����ϵ��

 ��
��