��Ŀ����
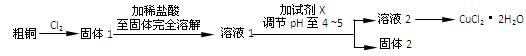
��6�֣��Ȼ�ͭ��һ�ֹ㷺�����������ϡ�ľ�ķ������ȵĻ�����Ʒ��ij�о�С���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���壨CuCl2��2H2O����
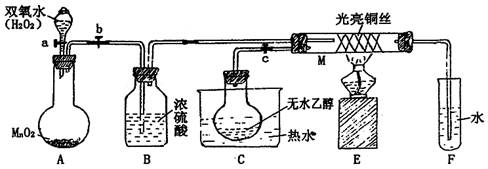
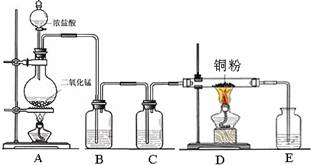
��1��ʵ���Ҳ�������ͼ��ʾ��װ�ã��ɽ���ͭ��Cl2��Ӧת��Ϊ�� ��1�����������ͼг�װ������ȥ����
��1�����������ͼг�װ������ȥ����

������A�������� ��
��װ��B�з�����Ӧ�����ӷ���ʽ�� ��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCl��װ�ã�����Ϊ�Ƿ��Ҫ����ǡ��� ��
�� NaOH��Һ������
��2���Լ�X���ڵ���pH�Գ�ȥ���ʣ� X��ѡ�������Լ��еģ�����ţ� ��
a��NaOH b��NH3��H2O c��CuO d��Cu2(OH) 2CO3 e��CuSO4

��1��ʵ���Ҳ�������ͼ��ʾ��װ�ã��ɽ���ͭ��Cl2��Ӧת��Ϊ��
 ��1�����������ͼг�װ������ȥ����
��1�����������ͼг�װ������ȥ����
������A�������� ��
��װ��B�з�����Ӧ�����ӷ���ʽ�� ��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCl��װ�ã�����Ϊ�Ƿ��Ҫ����ǡ��� ��
�� NaOH��Һ������
��2���Լ�X���ڵ���pH�Գ�ȥ���ʣ� X��ѡ�������Լ��еģ�����ţ� ��
a��NaOH b��NH3��H2O c��CuO d��Cu2(OH) 2CO3 e��CuSO4
��6�֣�
��1���ٷ�Һ©����MnO2 +4H++2Cl��=�� Mn2++ Cl2��+2H2O�۷�ܳ�ȥCl2��HCl
��2��c d ����1�֣�
��1���ٷ�Һ©����MnO2 +4H++2Cl��=�� Mn2++ Cl2��+2H2O�۷�ܳ�ȥCl2��HCl
��2��c d ����1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ___________________��
___________________�� N2O4(g) ��H �еĦ�H 0���>����<����
N2O4(g) ��H �еĦ�H 0���>����<����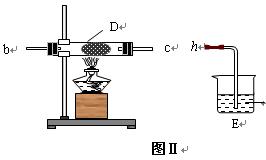
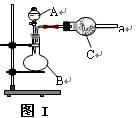
 ��װ���� ���������� ��
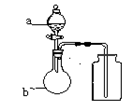
��װ���� ���������� �� ��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ ��
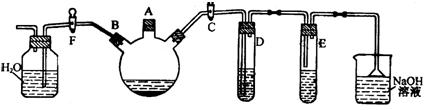
��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ �� ���������ʵ�顣��ʵ��װ������ͼ��ʾ��
���������ʵ�顣��ʵ��װ������ͼ��ʾ��