��Ŀ����
ͭ����Ҫ�Ľ������ϣ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ����ѧ����ʽΪ ���÷�Ӧ��������Ϊ________ ��
(2)��100 mL 18 mol��L��1 Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4��________mol.
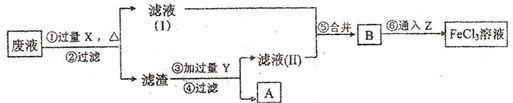
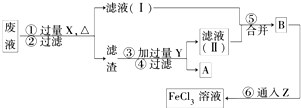
(3)���ӹ�ҵ����30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µ�FeCl3��Һ���������ʵ�����̣�

���������У������Լ��Ļ�ѧʽΪ��
X________________��Y____________��Z____________��
�ڢ���Ӧ�����ӷ���ʽΪ___________________________
��1��Cu2S + O2 ��2Cu + SO2 O2 ,.Cu2O ��2��0.9
��3�� X Fe Y HCl Z Cl2 2Fe 2+ + Cl2 == 2Fe 3+ + 2Cl-
����������1���������ʵ���ɿ�֪���������ͭ���⣬����SO2���ɣ����Է�Ӧ�ķ���ʽ��Cu2S + O2 ��2Cu + SO2�����ݷ���ʽ��֪����Ԫ�غ�ͭԪ�صĻ��ϼ۶��ǽ��͵ģ�������������O2 ��Cu2O��
��2�������ڷ�Ӧ�����У������Ũ�����ͣ���ϡ�����ͭ�Dz���Ӧ�ģ����Ա���ԭ������С��0.9mol��
��3����Һ�л�ԭ�Ȼ�ͭ���Ȼ������Ȼ��������������ȼ��������������û���ͭ����X�����������������������ڽ���������м��������ܽ��������ˡ�ϴ�Ӹ��T�õ�ͭ�����Y�����ᡣҪ���Ȼ���������Ȼ���������Ҫͨ����������Z����������Ӧ�����ӷ���ʽ��2Fe 2+ + Cl2 == 2Fe 3+ + 2Cl-��