��Ŀ����
��10�֣�A��B��C��D��E���������к���ͬһ��Ԫ�أ����ת����ϵ����ͼ��ʾ��
��1�����A�ǵ���ɫ���壬
��д��B��E�Ļ�ѧʽ��B��������E���� ����
��C��D�Ļ�ѧ����ʽΪ_____________________��
�۽�����������Cͨ����BaCl2��Һ�У�����˵����ȷ����___________������ĸ��ţ���
a����Һû�����Ա仯
b����Һ�г��ְ�ɫ����
c��������ͨ��Cl2��NH3����Һ�����ְ�ɫ����
��2�����A����ɫ���壺
D��E�Ļ�ѧ����ʽΪ���������������� ��
E��C�����ӷ���ʽΪ���������������� ��
��1С���2�֣�����ÿ��2�֣�
��1����H2S��H2SO4 ��2SO2+O2 2SO3 �� a c
��2��3NO2+H2O=2HNO3+NO 3Cu��8HNO3��ϡ��=3Cu(NO3)2��2NO����4H2O
����:

��ϰ��ϵ�д�
�����Ŀ



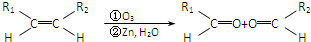

 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������