��Ŀ����
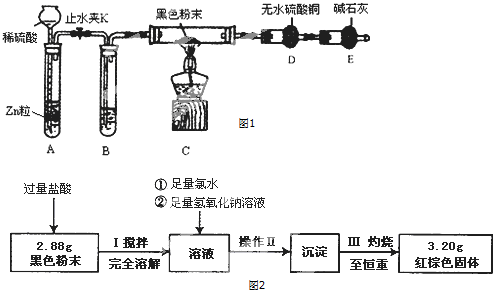
16��ij��ȤС��ͬѧ��ʵ�����ü���1-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������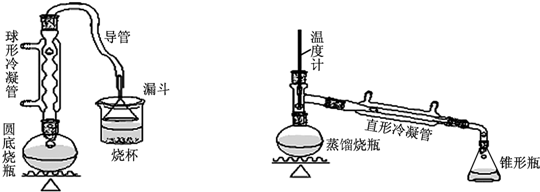
��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���Ǽȿ������ճ�֣��ֿ��Է�ֹ������
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ����ab��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С�-CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô��
��4��Ϊ�˽�һ���ᴿ1-�嶡�飬��С��ͬѧ�������л�����й����������
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
���� ��1�������ﺬ��HBr�ȣ�������
��2��l-������Ũ����������£��ɷ������Ӽ�ͷ�������ˮ����Ũ�������ǿ�����ԣ�
��3�����ݺ�������ǵ����ã����з��ӽṹ�ͻ�ѧ��ɷ�����
��4������ʱ��Ӧ�ﵽ1-�嶡��ķе㣮
��� �⣺��1������һ��©�����������Һ��ĽӴ�����������ܳ�ֱ������ҷ�ֹ������
�ʴ�Ϊ���ȿ������ճ�֣��ֿ��Է�ֹ������
��2��l-������Ũ����Ĵ������·�����������ˮ��ȡ��ϩ�����Ӽ���ˮ�õ��ѣ��������Ӽ���ˮ�γ���CH3CH2CH2CH2OCH2CH2CH2CH3����������ˮ��������ϩCH2=CHCH2CH3��ͬʱ�������ӱ�Ũ�����������嵥�ʣ�
�ʴ�Ϊ��ab��
��3������������������ʶԲ�ͬ�����ĺ��������������ԣ����з��ӽṹ�ͻ�ѧ��ɷ���������CH3CH2CH2CH2BrҲ����-CH2CH2CH2CH3�����Բ���ͨ�������������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3����
�ʴ�Ϊ��������������1-�嶡��Ҳ����-CH2CH2CH2CH3��
��4���ᴿ1-�嶡�飬�ռ��������Ϊ1-�嶡�飬�����뽫1-�嶡������������Һ�����������¶ȴ���е㣬
�ʴ�Ϊ��101.6�森
���� ������Ҫ������1-�嶡�����ȡʵ�飬Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬������Ӧԭ���ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | Cl-�Ľṹʾ��ͼ�� | |
| B�� | �۱�ϩ�Ľṹ��ʽ��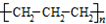 | |
| C�� | ���������ĵ���ʽ�� | |
| D�� | ����������ص��뷽��ʽ��KHSO4$\frac{\underline{\;����\;}}{\;}$ K++H++SO42- |
| A�� | ϴ�ӳ����ķ�����ֱ����������м�����������ˮ��Ȼ����ˮ��Ȼ���� | |
| B�� | �ñ���ȡ��ˮ�е���ʱ������ı���Һ�ӷ�Һ©���¿ڷų� | |
| C�� | ��100ml��Ͳ��ȡ9.5 mL��Һ�� | |
| D�� | ��ɫ��Ӧ�У��۲���ɫ��Ӧ���Ƚ���˿���뵽ϡ�����У�Ȼ���ٴ����� |
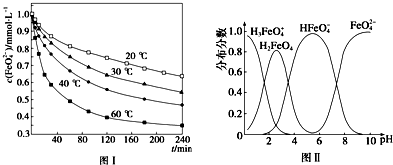
��֪25�棬�������ʵ��ܶȻ����������
| ���� | Mn��OH��2 | Co��OH��2 | Ni��OH��2 | MnS | CoS | NiS |
| Ksp | 2.1��10-13 | 3.0��10-16 | 5.0��10-16 | 1.0��10-11 | 5.0��10-22 | 1.0��10-22 |
��2��������1����Ҫ�ɷ�ΪFe��OH��3���ѧʽ������֪MnO2������Ϊ����������õ��óɷ����漰�����ӷ���ʽΪ2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��Fe3++3NH3�qH2O=Fe��OH��3��+3NH4+��
��3��������У����ӣ�NH4��2S��Ũ�Ȳ��˹����ԭ��������NH4��2S��Ũ�ȹ�����MnS��������ɲ�Ʒ��ʧ��
��4����Һ2�У�c��Co2+����c��Ni2+��=5��1��
��5��������Ϊa kg��̼���̿��������̴�����õ�����Mn b kg����ÿһ����������ȫ������1Ϊ���������Ϊc kg����ԭ̼���̿���MnCO3����������Ϊ$\frac{��b-\frac{c}{107}��\frac{1}{2}��55����\frac{115}{55}}{a}$��100%�����ú�a��b��c��ʽ�ӱ�����軯��
| A�� | 1molFe���ڹ������ᣬ����ת����Ϊ2NA | |
| B�� | 1molN2��4molH2��Ӧ���ɵ�NH3������Ϊ2NA | |
| C�� | ��״���£�2.24LCCl4���еĹ��ۼ���Ϊ0.4NA | |
| D�� | 14g��ϩ�ͱ�ϩ��������е���ԭ����Ϊ2NA |