��Ŀ����
����Ŀ����ѧʽΪC2H6O�Ļ�����A�����������ʣ�A��Na�D��������������
A��CH3COOH![]() ����ζ�IJ���
����ζ�IJ���
��1������������Ϣ���Ըû�������������ж���( )��
A��һ�����С�OH B��һ�����С�COOH C��AΪ�Ҵ� D��AΪ��ȩ
��2����A���������Ϊ75%��ˮ��Һ��������_______________________��
��3��A���Ʒ�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ���Ľṹ��ʽΪ��________��
��5��д��A�����ڴ������������¼��Ⱥ�������Ӧ�Ļ�ѧ����ʽ��___________________��
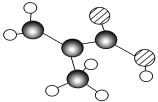
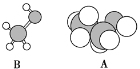
��6����ͼ��B���ӵ����ģ�ͺ�A���ӵı���ģ�ͣ���A��B�Ĺ�ϵ��ͬϵ���B�Ľṹ��ʽ_____��
���𰸡���1��AC ��2�֣�
��2��ҽ��������
��3��2Na��2CH3CH2OH��2CH3CH2ONa��H2�� ��2�֣�
��4��CH3COOCH2CH3 ��2�֣�
��5��2CH3CH2OH��O2![]() 2CH3CHO��2H2O ��2�֣� ��6�� CH3OH
2CH3CHO��2H2O ��2�֣� ��6�� CH3OH
��������
�����������1��A�����Ʒ�Ӧ�����������������ᷢ��������Ӧ�������A�ķ���ʽC2H6O��֪A���Ҵ�����ԭ��OH����ѡAC��
��2�����Ҵ����������Ϊ75%��ˮ��Һ��������ҽ����������
��3���Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na��2CH3CH2OH��2CH3CH2ONa��H2����
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ����������������ṹ��ʽΪCH3COOCH2CH3��
��5���Ҵ������ڴ������������¼��Ⱥ�������Ӧ�Ļ�ѧ����ʽΪ
2CH3CH2OH��O2![]() 2CH3CHO��2H2O��
2CH3CHO��2H2O��
��6������B���ӵ����ģ�ͺ�A���ӵı���ģ�ͣ���A��B�Ĺ�ϵ��ͬϵ���Bһ���Ǽ״����ṹ��ʽΪCH3OH��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������Dz��ֶ������еڶ�����������Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۡ�
Ԫ�ش��� | �� | �� | �� | �� | �� | �� | �� | �� |
ԭ�Ӱ뾶��nm�� | 0.186 | 0.160 | 0.152 | 0.143 | 0.110 | 0.099 | 0.075 | 0.074 |
��Ҫ���ϼ� | ��1 | ��2 | ��1 | ��3 | ��5����3 | ��7����1 | ��5����3 | ��2 |
�ش��������⣺
��1������Ԫ�����ڱ��е�λ���ǣ����ڡ��壩 ��
��2��8��Ԫ�ص�����������ˮ�����У�������ǿ���� ���ѧʽ����
��3��Ԫ�������������ֱ��γɵļ���̬�⻯���У����ĵ���ʽΪ ���ȶ�����ǿ���⻯��ĽṹʽΪ ��
��4��д��������������������Ӧ��ˮ����֮�䷢����Ӧ�����ӷ���ʽ ��
��5���õ���ʽ��ʾԪ���������γɻ�����Ĺ��� ��