��Ŀ����
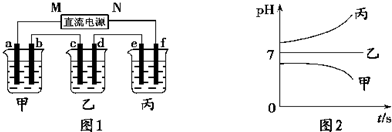
��A��B��C���ֵ������Һ�ֱ�װ�������ձ��У�����ʯī�缫����������ͼ��ʾ��ʽ�ڵ�·�����ӡ��պϿ���S��ø�֧·����I����I��(����I����С)������ȥB����ø�����IA����IC;����ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·�����ͨ��A��B�����Һ�ĵ�������ǰͨ��A��Һ�ĵ�������Դ�С��ϵΪ��IAB����IA�� 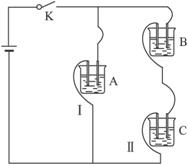
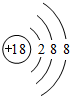
��֪A��B��C�ֱ�ѡ��������Һ��0.1 mol��L-1���ᡢ0.1 mol��L-1���ᡢ0.1 mol��L-1 NaCl��Һ��0.1 mol��L-1 NaOH��Һ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7��
����������⣺
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��
A.______________��B.______________��C.______________��
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C��________����A��B��C�ֱ��Ե��������������ϣ������________��ϵĻ��Һ�У�ˮ�ĵ���̶����ѡ��A��B��C�ش𣩡�
��1��0.1 mol��L-1���� 0.1 mol��L-1��ˮ �����NaCl��Һ��NaOH��Һ
��2��NaOH��Һ AB
�������ڵ�ѹ���ʱ������Ũ��Խ������Һ�ĵ�����Խǿ����·�ĵ���Խ�պ�S��I����I������ȥB��IA����IC��˵��A��B�����������Һ��C��ǿ�������Һ��IAB����IA��˵��A��B��Ϻ�����ǿ����ʡ���֪��Һ��ֻ�д�����Һ�Ͱ�ˮ�����������Һ������ΪA��Һ��pH��7����A�Ǵ�����Һ��B�ǰ�ˮ�����ڴ������ˮ�⣬����Һ��ˮ�ĵ���̶����

��ϰ��ϵ�д�
�����Ŀ