题目内容
下列离子方程式中,正确的是( )
| A.NH4HSO3溶于少量NaOH溶液中: HSO3-+OH- = SO32-+H2O |
| B.酸性KMnO4溶液与双氧水反应: 2MnO4-+ 7H2O2 + 6H+= 2Mn2+ + 6O2↑+ 10H2O |
| C.向氯化银悬浊液中滴入饱和碘化钾溶液:Ag++ I-= AgI ↓ |
| D.标准状况下672mL Cl2通入100mL0.1 mol·L-1FeBr2溶液中: |
A
解析试题分析:A、HSO3?先与OH?反应,正确;B、离子方程式没有配平,根据化合价升降法配平可得:2MnO4?+5H2O2+6H+=2Mn2++5O2↑+8H2O,错误;C、AgCl为难溶物质,不能拆分为Ag+,错误;D、Cl2为0.03mol,FeBr2为0.01mol,Cl2足量,Fe2+与Br?完全被氧化:2Fe2++4Br-+3Cl2=2Fe3++2Br2+6Cl-,错误。
考点:本题考查离子方程式。

练习册系列答案
相关题目
下列离子方程式正确的是( )
| A.碳酸钙溶于醋酸:CaCO3+2H+= Ca2++CO2↑ +H2O |
| B.Fe3O4溶于稀盐酸:Fe3O4+8H+=3Fe3++4H2O |
| C.用烧碱溶液吸收氯气:Cl2+2OH-=Cl-+ClO-+H2O |
| D.向硫酸亚铁溶液中加入用硫酸酸化的H2O2溶液 : Fe2++H2O2+2H+=Fe3++4H2O |
下列各组离子在指定条件下,一定能大量共存的是
| A.pH为1的无色溶液:K+、Fe2+、SO32-、Cl- |
| B.能使碘化钾淀粉试纸变蓝的溶液:Na+、NH4+、S2-、Br- |
| C.水电离出的c(H+)=10-12mol/L的溶液:Ba2+、Na+、NO3-、Cl- |
| D.加入铝条有氢气放出的溶液:Na+、NH4+、HCO3-、SO42- |
有一混合物的水溶液,只可能含有以下离子中的若干种:K+、NH4+、Cl-、Mg2+、Ba2+、CO32-、SO42-,现取三份均为100mL溶液进行如下实验:
(1)第一份加入AgNO3溶液有沉淀产生
(2)第二份加足量NaOH溶液加热后,收集到气体0.04mol
(3)第三份加足量BaCl2溶液后,得干燥沉淀6.27g,经足量盐酸洗涤、干燥后,沉淀质量为2.33g。根据上述实验,以下推测正确的是( )
| A.K+一定存在 | B.100mL溶液中含0.01mol CO32- |
| C.Cl-一定存在 | D.Ba2+一定不存在,Mg2+可能存在 |
将由NaOH、BaCl2、Al2(SO))3三种固体组成的混合物溶于足量的水中,充分溶解,向混合溶液中滴加1mol·L-1的稀硫酸,加入稀硫酸的体积与生成沉淀的质量关系如图所示。下列有关判断不正确的是( )
| A.AB段发生反应的离子方程式为:Ba2++SO42-=BaSO4↓ |
| B.E点对应横坐标稀硫酸的体积为70 ml |
| C.D点表示的沉淀的化学式为Al(OH)3、BaSO4 |
| D.E点沉淀比A点沉淀质量大2.33g |
下列相关反应的离子方程式书写正确的是( )
| A.氢氧化铁溶于氢碘酸:Fe(OH)3 +3H+ =Fe3++3H2O |
| B.NaHCO3溶液中加入过量的Ba(OH)2溶液:2HCO3—+Ba2++2OH— = BaCO3â +2H2O+CO32— |
| C.NH4Al(SO4)2溶液中加入Ba(OH)2溶液使SO42-完全沉淀:Al3++2SO42-+2Ba2++4OH-=AlO2-+2BaSO4↓+2H2O |
| D.向含有0.4 mol FeBr2的溶液中通入0.1 mol Cl2反应:2Fe2++Cl2=2Fe3+ +2Cl- |
下列各表述与示意图一致的是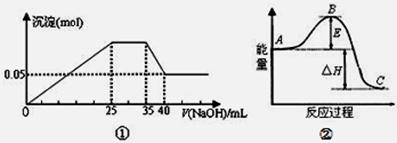
| A.图①表示向含Mg2+、Al3+、NH4+的盐溶液中滴加NaOH溶液时,沉淀的量与NaOH的体积的关系图。则三种离子的物质的量之比为:n(Mg2+):n(Al3+):n( NH4+)=2:3:2 |
| B.图①中使用的NaOH的浓度为2mol/L |
| C.图②中曲线表示某反应过程的能量变化。若使用催化剂,B点会降低 |
| D.图②中物质A反应生成物质C,△H>0; |
下列离子方程式书写正确的是:
| A.碳酸氢钙溶液中加入过量的NaOH溶液: Ca2++HCO3―+OH―=CaCO3↓+H2O |
| B.NaHSO4溶液与Ba(OH)2溶液混合后显酸性:Ba2++OH-+H++SO42-=BaSO4↓+H2O |
| C.FeI2溶液中通入少量Cl2:2 Fe2+ + Cl2="2" Fe3+ + 2Cl- |
| D.向NH4Al(SO4)2溶液中滴入Ba(OH)2溶液恰使SO42-沉淀完全NH4++Al3++2Ba2++2SO42-+4OH- =Al(OH)3↓+NH3·H2O+2BaSO4↓ |
下列各式中,属于正确的电离方程式的是
| A.HCO3- = CO32- + H+ | B.HCO3- +OH- = H2O + CO32- |
| C.NH3+ H+ = NH4+ | D.NH3·H2O 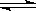 NH4+ + OH- NH4+ + OH- |