ћвƒњƒЏ»Ё
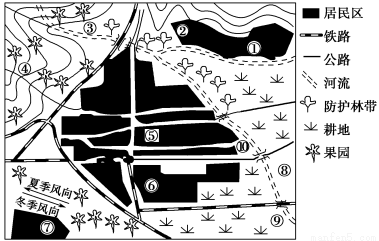
ґЅƒ≥≥« –µƒ≥« –≤ЉЊ÷ Њ“вЌЉ£ђїЎір”–єЎќ ћв£Ї
Ґў√ЇћњїщµЎ°°ҐЏіу–ЌЅтЋб≥І°°Ґџ„‘јіЋЃ≥І°°Ґ№Ѕ∆—ш‘Ї°°ҐЁіу–Ќ…ћ≥°°°Ґё ≥∆Ј≥І°°Ґяїѓє§≥І°°Ґа‘м÷љ≥І°°ҐбќџЋЃі¶јн≥І°°ҐвЄяµ»‘Ї–£
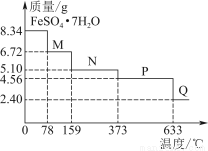
£®1£©Є√≥« –≤ЉЊ÷ «ЈсЇѕјн£њ«лЈ÷ќц‘≠“т°£
£®2£©ЌЉ÷–ҐЏі¶Ј÷≤Љіу–ЌЅтћъњу£®÷ч“™≥…Ј÷ «FeS2£©°£–і≥ц÷∆»°ґю—хїѓЅт°Ґ»э—хїѓЅтµƒїѓ—ІЈљ≥ћ љ°£
£®3£©љ”і•Ј®÷∆ЅтЋбµƒќ≤∆ш÷–їєЇђ”–…ўЅњµƒґю—хїѓЅт£ђ»зєы≈≈»ліу∆ш£ђЊЌїб‘м≥…їЈЊ≥ќџ»Њ°£ƒг»ѕќ™Є√∆у“µ”¶Є√≤…”√ ≤√іЈљЈ®їЎ ’°Ґјы”√ќ≤∆ш÷–µƒґю—хїѓЅт£ђ–і≥ц”–єЎЈі”¶µƒїѓ—ІЈљ≥ћ љ°£
£®4£©Є√ –Њ≠Љ√ЈҐіп£ђ√њћм”–іуЅњµƒ∆ы≥µ≈≈Ј≈µ™—хїѓЇѕќп°ҐћЉ«вїѓЇѕќп£ђ“‘Љ∞∆у“µ°ҐЉ“Ќ•¬ѓ‘о≈≈Ј≈іуЅњµƒґю—хїѓЅт°Ґґю—хїѓћЉµ»£ђЄ√ –”¶≤…»°ƒƒ–©іл ©Јј÷ќіу∆шќџ»Њ£њ
Љыљвќц£®ЇѕјнЉіњ…£©°£
°Њљвќц°њ£®1£©√ЇћњїщµЎ°Ґіу–ЌЅтЋб≥І°Ґїѓє§≥І≤ЉЊ÷Їѕјн£ђјн”… «Ћь√«ґЉљ”љь‘≠ЅѕїщµЎ£ђ«“‘Џіє÷±ЈзѕтµƒљЉЌв°£„‘јіЋЃ≥І°ҐЅ∆—ш‘Ї°Ґіу–Ќ…ћ≥°°Ґ ≥∆Ј≥І≤ЉЊ÷Їѕјн£ђ„‘јіЋЃ≥І‘ЏЇ”Ѕч…ѕ”ќ£ђ≤ї №їт…ў №ќџ»Њ°£‘м÷љ≥І≤їЇѕјн£ђјн”… «Ћьќї”ЏѕƒЉЊЈзµƒ…ѕЈзµЎіш£ђ«“јлЊ”√с«ш°ҐЄяµ»‘Ї–£љѕљь£ђіу∆шќџ»Њ—ѕ÷Ў°£ћъ¬Јі©єэ –«ш≤їЇѕјн£ђ“тќ™‘л…щќџ»Њ°Ґіу∆шќџ»Њ—ѕ÷Ў°£ќџЋЃі¶јн≥І‘ЏЇ”Ѕчѕ¬”ќ£ђЇѕјн°£
£®2£©4FeS2£Ђ11O2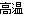 2Fe2O3£Ђ8SO2°Ґ 2SO2£ЂO2
2Fe2O3£Ђ8SO2°Ґ 2SO2£ЂO2 2SO3
2SO3
£®3£©∞±ќь ’Ј®£ЇSO2£Ђ2NH3£ЂH2O=£®NH4£©2SO3°Ґ£®NH4£©2SO3£ЂH2SO4=£®NH4£©2SO4£ЂSO2°ь£ЂH2O£ђ’в—щµ√µљµƒSO2ЇђЅњљѕЄя£ђњ…ЈµїЎ”√„ч‘≠Ѕѕ°£
£®4£©Є√ –”¶≤…»°µƒіл © «£ЇҐўіуЅ¶÷≤ ч‘мЅ÷£їҐЏѕё÷∆іуЅњ∆ы≥µљЂћЉ«вїѓЇѕќп°Ґµ™—хїѓЇѕќп≈≈»ліу∆ш£їҐџ≤…”√њ∆—ІЉЉ хґ‘ЅтЋб≥І°Ґїѓє§≥І°Ґ‘м÷љ≥І°Ґ√ЇћњїщµЎµƒЈѕ∆шљш––їЎ ’і¶јн£їҐ№іі‘мћхЉю£ђљЂ‘м÷љ≥І«®“∆µљ‘ґјл –«шµƒљЉЌвµ»µ»°£

‘Џ2 L√№±’»Ё∆чƒЏ,800 °ж ±Јі”¶2NO(g)+O2(g) 2NO2(g)ћеѕµ÷–,n(NO)Ћж ±Љдµƒ±дїѓ»зѕ¬±н:
2NO2(g)ћеѕµ÷–,n(NO)Ћж ±Љдµƒ±дїѓ»зѕ¬±н:
±Љд/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |

(1)…ѕЌЉ±н ЊNO2µƒ≈®ґ»±дїѓµƒ«ъѕя «°°°°°°°°°£”√O2±н Њі”0~2 sƒЏЄ√Јі”¶µƒ∆љЇвЋў¬ v=°°°°°°°°°£
(2)ƒ№Ћµ√чЄ√Јі”¶“—іпµљ∆љЇв„іћђµƒ «°°°°°°°°°£
a.v(NO2)=2v(O2)°°°°b.»Ё∆чƒЏ—є«њ±£≥÷≤ї±д
c.vƒж(NO)=2v’э(O2)°°°°d.»Ё∆чƒЏ√№ґ»±£≥÷≤ї±д