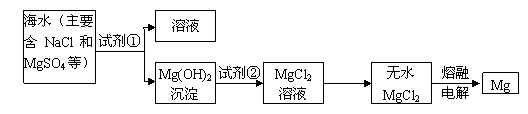��Ŀ����
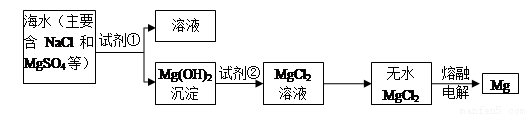
I��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ���Ҫ�������£�

��1��Ϊ��ʹMgSO4ת��ΪMg��OH��2���Լ��ٿ���ѡ��
��2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ�����
��3���Լ���ѡ��
��4����ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ
II�����ͼ������������Ĵ������ʣ��ش��������⣺
��1�����ڳ��³�ѹ�³�
��2�����³�ѹ����ֱ̬��������̼ԭ������
��3�����ͼ����к�̼���ϸߵ���
��4��д��������������ȼ�յĻ�ѧ����ʽ
��5��д�������嵥�ʷ�Ӧ�Ļ�ѧ����ʽ

��1��Ϊ��ʹMgSO4ת��ΪMg��OH��2���Լ��ٿ���ѡ��
��������
��������
��ҪʹMgSO4��ȫת��Ϊ�����������Լ�����ӦΪ����
����
����2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ�����
����
����
����3���Լ���ѡ��
����
����
��д���䷴Ӧ�����ӷ���ʽMg��OH��2+2H+=Mg2++2H2O
Mg��OH��2+2H+=Mg2++2H2O
����4����ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ
MgCl2�����ڣ�
Mg+Cl2��
| ||
MgCl2�����ڣ�
Mg+Cl2��
��
| ||
II�����ͼ������������Ĵ������ʣ��ش��������⣺
��1�����ڳ��³�ѹ�³�
Һ
Һ
̬����2�����³�ѹ����ֱ̬��������̼ԭ������
4
4
����3�����ͼ����к�̼���ϸߵ���
��
��
����4��д��������������ȼ�յĻ�ѧ����ʽ
CH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
�����������ʵ����ı��ͼ���ֱ�ȼ�գ��������ϴ����
| ||
��
��
����5��д�������嵥�ʷ�Ӧ�Ļ�ѧ����ʽ
C6H6+Br2
C6H5Br+HBr
| FeBr3 |
C6H6+Br2
C6H5Br+HBr
��| FeBr3 |
������I����1��Ϊ��ʹMgSO4ת��ΪMg��OH��2�����������ʺ��������������Ҳ������µ��������ӣ�ҪʹMgSO4��ȫת��Ϊ�����������Լ�����ӦΪ������
��2�����벻���Թ������Һ�ķ����ǹ��ˣ�
��3�������������þ��Ӧ�����Ȼ�þ��
��4����������Ȼ�þ����������þ��
II����1�����ڳ��³�ѹ�³�Һ̬��
��2�����³�ѹ����ֱ̬��������̼ԭ������4��
��3�����������ʽ�жϺ�̼���ߵͣ�
��4������ȼ�����ɶ�����̼��ˮ�����ݷ���ʽ�ж���������������С��
��5�����廯�������������£�����Һ�巢��ȡ����Ӧ��
��2�����벻���Թ������Һ�ķ����ǹ��ˣ�
��3�������������þ��Ӧ�����Ȼ�þ��
��4����������Ȼ�þ����������þ��
II����1�����ڳ��³�ѹ�³�Һ̬��
��2�����³�ѹ����ֱ̬��������̼ԭ������4��
��3�����������ʽ�жϺ�̼���ߵͣ�
��4������ȼ�����ɶ�����̼��ˮ�����ݷ���ʽ�ж���������������С��
��5�����廯�������������£�����Һ�巢��ȡ����Ӧ��
����⣺��1��ʹMgSO4ת��ΪMg��OH��2��Ӧѡ����ת���в������µ����ӣ����Լ���ѡ��NaOH��Ϊ��ʹþ������ȫת���������Լ��ٵ���Ӧ������
�ʴ�Ϊ��NaOH��������
��2�����������ڲ�����ˮ�Ĺ����Һ�壬������þ������ˮ�����Է���õ�Mg��OH��2�����ķ����ǹ��ˣ�
�ʴ�Ϊ�����ˣ�
��3����������þ���Ȼ�þ��������þ�����ᷴӦ�õ��Ȼ�þ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O���������ӷ���ʽΪMg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ�����Mg��OH��2+2H+=Mg2++2H2O��
��4���Ȼ�þ���Ϊ�ֽⷴӦ������Ϊþ�����������Ե�ⷽ��ʽΪ��MgCl2�����ڣ�
Mg+Cl2�����ʴ�Ϊ��MgCl2�����ڣ�
Mg+Cl2����
II����1�����ڳ��³�ѹ�³�Һ̬���ʴ�Ϊ��Һ��
��2�����³�ѹ����ֱ̬��������̼ԭ������4���ʴ�Ϊ��4��
��3�����������ʽ�жϺ�̼���ߵͣ�����ķ���ʽ�����ʽ����CH4���������ʽΪCH�����к�̼���ߣ��ʴ�Ϊ������
��4������ȼ�����ɶ�����̼��ˮ����Ӧ����ʽΪ��CH4+2O2
CO2+2H2O����ȼ�շ���ʽΪ��C6H6+7.5O2
6 CO2+3H2O�������ʵ����ı��ͼ���ֱ�ȼ�գ��������ϴ���DZ����ʴ�Ϊ��CH4+2O2
CO2+2H2O������
��5�����廯�������������£�����Һ�巢��ȡ����Ӧ����Ӧ����ʽΪ��C6H6+Br2
C6H5Br+HBr��
�ʴ�Ϊ��C6H6+Br2
C6H5Br+HBr��
�ʴ�Ϊ��NaOH��������
��2�����������ڲ�����ˮ�Ĺ����Һ�壬������þ������ˮ�����Է���õ�Mg��OH��2�����ķ����ǹ��ˣ�
�ʴ�Ϊ�����ˣ�
��3����������þ���Ȼ�þ��������þ�����ᷴӦ�õ��Ȼ�þ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O���������ӷ���ʽΪMg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ�����Mg��OH��2+2H+=Mg2++2H2O��
��4���Ȼ�þ���Ϊ�ֽⷴӦ������Ϊþ�����������Ե�ⷽ��ʽΪ��MgCl2�����ڣ�
| ||
| ||
II����1�����ڳ��³�ѹ�³�Һ̬���ʴ�Ϊ��Һ��
��2�����³�ѹ����ֱ̬��������̼ԭ������4���ʴ�Ϊ��4��
��3�����������ʽ�жϺ�̼���ߵͣ�����ķ���ʽ�����ʽ����CH4���������ʽΪCH�����к�̼���ߣ��ʴ�Ϊ������
��4������ȼ�����ɶ�����̼��ˮ����Ӧ����ʽΪ��CH4+2O2
| ||
| ||
| ||
��5�����廯�������������£�����Һ�巢��ȡ����Ӧ����Ӧ����ʽΪ��C6H6+Br2
| FeBr3 |
�ʴ�Ϊ��C6H6+Br2
| FeBr3 |
���������⿼���˺�ˮ��Դ���ۺ����ú��л���ѧ�����ݽ����Ļ�����ȷ��������ұ��������ע����Ӽ���ѡȡ��Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ