��Ŀ����
19����1�������£���0.15mol•L-1ϡ����V1 mL��0.1mol•L-1 NaOH��ҺV2 mL��ϣ�������Һ��pHΪ1����V1��V2=1��1������Һ����仯���Բ��ƣ���2�������£�ijˮ��Һ�д��ڵ������У�Na+��A-��H+��OH-���������⣬�ش��������⣺
������0.1mol•L-1 HA��Һ��0.1mol•L-1 NaOH��Һ�������϶��ã�����Һ��pH��7��
������ҺpH��7����c��Na+����c��A-���������Ǹ��ݵ���غ㣬c��H+��+c��Na+��=c��OH-��+c��A-��������c��OH-����c��H+������c��Na+����c��A-����
������Һ��pH=3��HA��ҺV1 mL��pH=11��NaOH��ҺV2 mL��϶��ã�������˵����ȷ����AD����ѡ����ţ���
A������Ӧ����Һ�����ԣ���c��H+��+c��OH-��=2��10-7 mol•L-1
B����V1=V2����Ӧ����Һ��pHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2��
���� ��1��pH=1�Ļ����Һ��c��H+��=0.1mol/L�������Һ��c��H+��=$\frac{0.15��2��{V}_{1}-0.1��{V}_{2}}{{V}_{1}+{V}_{2}}$mol/L=0.1mol/L���ݴ˼���V1��V2��
��2��������0.1mol•L-1 HA��Һ��0.1mol•L-1 NaOH��Һ�������϶��ã����HA�����ᣬ����ǡ�÷�Ӧ����ǿ�������Σ���Һ�ʼ��ԣ��������ǿ�ᣬ����ǡ�÷�Ӧ����ǿ��ǿ���Σ���Һ�����ԣ�
������ҺpH��7��˵��c��H+����c��OH-�������ݵ���غ��ж�c��Na+����c��A-����Դ�С��
������Һ��pH=3��HA��ҺV1 mL��pH=11��NaOH��ҺV2 mL��϶��ã�
A������Ӧ����Һ�����ԣ���HA��ǿ�ᣬ�����£���Һ��c��H+��=c��OH-��=1��10-7 mol•L-1��
B����V1=V2���������ǿ�ᣬ�����Һ�����ԣ�����������ᣬ�����Һ�����ԣ�
C������Ӧ����Һ�����ԣ�����Һ�������������Һ��
D�������Һ�ʼ��ԣ�����Һ�����Ǽ������Һ��Ҳ����ֻ������Һ��
��� �⣺��1��pH=1�Ļ����Һ��c��H+��=0.1mol/L�������Һ��c��H+��=$\frac{0.15��2��{V}_{1}-0.1��{V}_{2}}{{V}_{1}+{V}_{2}}$mol/L=0.1mol/L��V1��V2=1��1���ʴ�Ϊ��1��1����
��2��������0.1mol•L-1 HA��Һ��0.1mol•L-1 NaOH��Һ�������϶��ã����HA�����ᣬ����ǡ�÷�Ӧ����ǿ�������Σ���Һ�ʼ��ԣ���pH��7���������ǿ�ᣬ����ǡ�÷�Ӧ����ǿ��ǿ���Σ���Һ�����ԣ�����pH=7��
�ʴ�Ϊ���ݣ�
������ҺpH��7��˵��c��H+����c��OH-�������ݵ���غ��c��Na+����c��A-����
�ʴ�Ϊ���������ݵ���غ㣬c��H+��+c��Na+��=c��OH-��+c��A-��������c��OH-����c��H+������c��Na+����c��A-����
��A���������Һ�����ԣ�����ҺM��c��H+��=c��OH-��=2��10-7 mol•L-1��������ҺM��c��H+��+c��OH-��=2��10-7 mol•L-1������ȷ��
B��������������ȣ��������ǿ�ᣬ������Һ�����ԣ�����������ᣬ������Һ�����ԣ��ʴ���
C�������Һ�����ԣ�����Һ���������Һ���������ᣬ��Ũ�ȴ�����������Ũ�ȣ�����V1��һ������V2���ʴ���
D�������Һ�ʼ��ԣ�����Һ�����Ǽ������Һ��Ҳ����ֻ������Һ����V1һ��С��V2������ȷ��
��ѡAD��
���� ���⿼���������Һ�����жϣ�Ϊ��Ƶ���㣬�漰����Ũ�ȴ�С�Ƚϡ�pH�ļ��㣬���ؿ���ѧ�������жϼ������������ѵ��ǣ�2�����и�����Һ�����ȷ����Һ�����Դ�С����Ŀ�Ѷ��еȣ�

| A�� | ���۵ķ���ʽ��C12H22O11 | |
| B�� | ���ۿ��Է���������Ӧ | |
| C�� | ���ۿ���ֱ�Ӽ���ʳ�����Ƿ��е�Ԫ�� | |
| D�� | ��������Ȼ�߷��ӻ����� |
�ٳ����Ŀ��� �ھƾ� ��īˮ ��ϡ���� ������ͭ��Һ��
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �٢ۢ� | D�� | �ڢۢ� |
| A�� | ��������ڷ����ᴿҺ̬�л������ | |
| B�� | ��ϩ�ͼ������������Ȼ�̼��Һ���� | |
| C�� | �˴Ź�������ͨ�����ڷ����л������Է������� | |
| D�� | �������������ȡ����Ӧ��Ҫ���� |

| A�� | ͼʾʵ���з����˻�ѧ�仯 | |
| B�� | Һ��X��Һ��pH��7��Һ��Y��һ�ֺ�ɫ��״Һ�� | |
| C�� | ����Z��ȼ���ɻ�ԭCuO��Ҳ��ʹ��ˮ��ɫ | |
| D�� | �Թ�A�в���Ũ�İ��� |
| A�� | ��ѿ�Ǽ���ˮ�������ܷ���������Ӧ | |
| B�� | �����������£�CH3CO18OC2H5��ˮ�������CH3CO18OH��C2H5OH | |
| C�� | �����ͼȿ�ʹ������Ȼ�̼��Һ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ | |
| D�� | �øʰ���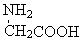 �ͱ����� �ͱ�����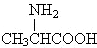 ���������γ�4�ֶ��� ���������γ�4�ֶ��� |
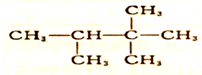 ��ϵͳ����Ϊ2��2��3-�������飮
��ϵͳ����Ϊ2��2��3-�������飮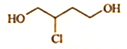 �ķ���ʽΪC4H9O2Cl��
�ķ���ʽΪC4H9O2Cl��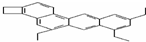 doggycene�ķ���ʽΪC26H26��
doggycene�ķ���ʽΪC26H26��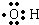 ��
��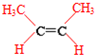 ��
��