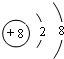��Ŀ����
����Ԫ��A��B��C��D��ԭ��������С��18��A��DΪͬ����Ԫ�أ�B��C��ͬһ���ڣ�A��Dԭ�Ӽ۵���Ϊ1��Cԭ�ӵ�������������Bԭ�ӵ���2�����������������Ǵ�����������2����A��B�ĵ����ڳ����¶������壬�����ڸ��������������2��1��ȫ��Ӧ���������ڳ�������Һ�壮��Һ����D�ĵ����ܼ��ҷ�Ӧ����A�ĵ��ʣ�������Һ�����̪�Ժ�ɫ��ͬʱ��Һ�к��к���ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӣ��ش��������⣺
(1)д��Ԫ�ط���A________��B________��C________��D________��
(2)д��B��C�ڸ�������ȫ��Ӧ������ķ���ʽ________������ʽ________���ṹʽ________��������________�Լ�(����ԡ��Ǽ��ԡ�)��ϵ�________���ӣ����ӵĹ���Ϊ________��
(3)�õ���ʽ��ʾB��D�ڸ������γɵĻ�����Ľṹ________���ж����еĻ�ѧ������________��
(4)д����A��B��C��D����Ԫ����ɵĻ��������ʽ________��
�𰸣�
������
������
|
����(1)H,O,C,Na ����(2)CO2, ����(3) ����(4)NaHCO3 |

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ