��Ŀ����
�����ʼ��仯���������������Ӧ�ù㷺����ش��������⣺
(1)�����ڿ����з���������ʴʱ�������ĵ缫��ӦʽΪ___________________________��
(2)����������Fe3��>Cu2�����Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽ��____________________________________________________________________��
(3)�����������������������ھ�ˮ����ԭ���� ������������������������������
(�����ӷ���ʽ��ʾ)����ʹ��ʱ��������������ʹ���Է�ˮ�е��������������ȥ����ԭ����___________________________�� ��������������������������������_��
(4)�������ǹ�ҵ��ұ������ԭ��֮һ����ԭ����Fe3O4��4CO3Fe��4CO2������1.5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����_________�� _��
(5)�±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����________(����ĸ)��
ѡ�� | ������ | ������ | �ж� |
A | ���ǵؿ��к�����ߵĽ���Ԫ�� | ������������ʹ�õĽ������� | ��ԣ���ԣ��� |
B | ����������ϡ���ᷴӦ�������� | �����������ܻ�ԭ�������õ��� | ��ԣ���ԣ��� |
C | �����ڹ���Ԫ�� | ��������ijЩ��������������� | �������ԣ��� |
D | �ڿ��������ı������γ����ܵ�����Ĥ | ��������������Ӧ | ��ԣ���ԣ��� |
(1)O2��2H2O��4e��===4OH��
(2)2Fe3����Cu===2Fe2����Cu2��
(3)Fe3����3H2O===Fe(OH)3(����)��3H�������Ի����У�H�������������ӵ�ˮ�⣬������������������
(4)12 mol��(5)B

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д������ʼ��仯����������������й㷺Ӧ�á���ش��������⣺
(1)������(FeS2)�����������ұ����������Ҫԭ�ϡ�����һ����ӦΪ3FeS2��8O2 6SO2��Fe3O4,3 mol
FeS2�μӷ�Ӧת��________mol
���ӡ�
6SO2��Fe3O4,3 mol
FeS2�μӷ�Ӧת��________mol
���ӡ�
(2)�Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
�Ӹ�ʴ��Һ���յõ�����ͭ����Ҫ���Լ�Ϊ
________________________________________________________________________��
(3)���������ƣ�������Ҳ����������������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ����____________________________________________________ ��
(4)�±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����________(����ĸ)��
|
ѡ�� |
������ |
������ |
�ж� |
|
A |
���ǵؿ��к�����ߵĽ���Ԫ�� |
������������ʹ�õĽ������� |
��ԣ���ԣ��� |
|
B |
����������ϡ���ᷴӦ�������� |
�����������ܻ�ԭ������������ |
�� �ԣ���ԣ��� |
|
C |
�����ڹ���Ԫ�� |
��������ij����������������� |
�� ������ԣ��� |
|
D |
���ڿ����б���������������Ĥ |
��������������Ũ���ᡢŨ���� |
�� �ԣ���ԣ��� |
�����ʼ��仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ��3FeS2��8O2 6SO2��Fe3O4��3 mol FeS2�μӷ�Ӧת��
mol���ӡ�
6SO2��Fe3O4��3 mol FeS2�μӷ�Ӧת��
mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽ ��
�Ӹ�ʴ��Һ���յõ�����ͭ����Ҫ���Լ�Ϊ__________ _______________��
��3�����������ƣ�������Ҳ����������������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ���� ��
��4���±��У��Գ���I��II����ȷ�Լ������������ϵ���ж϶���ȷ����__ __��������ĸ��
|
ѡ�� |
����I |
����II |
�ж� |
|
A |
���ǵؿ��к�����ߵĽ���Ԫ�� |
������������ʹ�õĽ������� |
I�ԣ�II�ԣ��� |
|
B |
����������ϡ���ᷴӦ�������� |
�����������ܻ�ԭ������������ |
I�ԣ�II�ԣ��� |
|
C |
�����ڹ��ɽ���Ԫ�� |
��������ij����������������� |
I����II�ԣ��� |
|
D |
���ڿ����б���������������Ĥ |
��������������Ũ���ᡢŨ���� |
I�ԣ�II�ԣ��� |
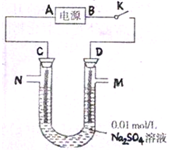 ��2011?������һģ���������ʼ��仯����������������Ӧ�ù㷺���������������������������ھ�ˮ����ԭ���ǣ������ӷ���ʽ��ʾ��
��2011?������һģ���������ʼ��仯����������������Ӧ�ù㷺���������������������������ھ�ˮ����ԭ���ǣ������ӷ���ʽ��ʾ�� Fe��OH��3+3H+
Fe��OH��3+3H+