��Ŀ����
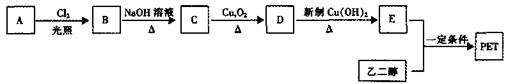
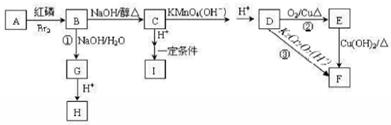
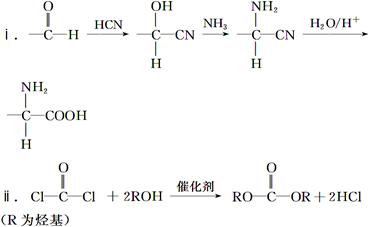
�ö���ȩ��ʳƷ�뻯ױƷ��ҵ�е��������Ӽ�����ҵ�Ͽ�ͨ���л�����ԭ��A�Ƶã���ϳ�·������ͼ��ʾ��
��֪���� 2CH2=CH2+O2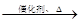 2CH3CHO
2CH3CHO
�� CH3CHO+CH3CHO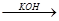 CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO
���������գ�
��1��A�Ľṹ��ʽ������ ��B�Ľṹ��ʽ������ ��
��1�������ϳ�·�������ڼӳɷ�Ӧ���� ����д��Ӧ��ţ���
��1��д����D����ö���ȩ�Ļ�ѧ��Ӧ����ʽ ��
��1��A��ͬ���칹���з��������������� �֡�
a����ʹFeC13(aq)����ɫ
b���ܷ����Ӿ۷�Ӧ
c��������ֻ��������Ϊ��λ��ȡ����
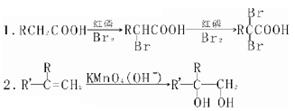
��1����Ҫд��֤��C�к���̼̼˫����ʵ�鲽�� ��
��1��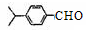 ��2�֣���
��2�֣���  ��2�֣�
��2�֣�
��2���٢� ��1�֡�2��
��3�� +2H2O ��2�֣�
+2H2O ��2�֣�
��4��8��2�֣�
��5��ȡ������������������������Cu(OH)2����Һ���������У���ȴ��ȡ�ϲ���Һ�ữ��������ˮ������ˮ��ɫ��֤������̼̼˫���������������𰸾����֣���2�֣�
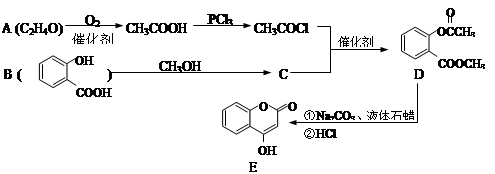
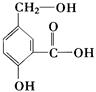
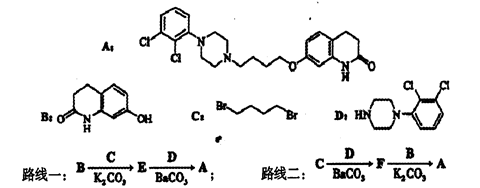
����������������ݲ�Ʒ���������ƣ����ѵõ���ص�ת����ϵ��
��1��A�Ľṹ��ʽ�� 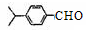 ��B�Ľṹ��ʽ��
��B�Ľṹ��ʽ�� ��
��
��2�������ϳ�·�������ڼӳɷ�Ӧ���Т٢� ��
��3��д����D����ö���ȩ�Ļ�ѧ��Ӧ����ʽ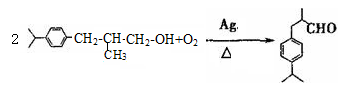 ����4��A��ͬ���칹���з���������������8�֡�
����4��A��ͬ���칹���з���������������8�֡�
��5����Ҫд��֤��C�к���̼̼˫����ʵ�鲽��ȡ������������������������Cu(OH)2����Һ���������У���ȴ��ȡ�ϲ���Һ�ữ��������ˮ������ˮ��ɫ��֤������̼̼˫������
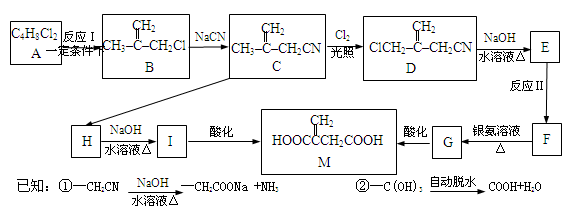
���㣺�����л��������֮���ת����ͬ���칹����д�ȡ�

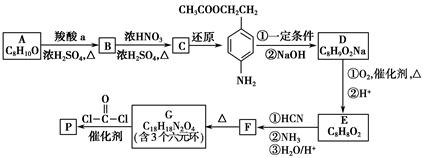
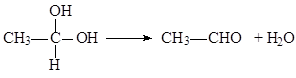


 �����ᣬ��B��ˮ����ﻥΪͬ���칹�塣H����FeCl3��Һ������ɫ��Ӧ���ұ����ϵ�һ�ȴ���ֻ��2�֡�д��������������������H�Ľṹ��ʽ��__________��
�����ᣬ��B��ˮ����ﻥΪͬ���칹�塣H����FeCl3��Һ������ɫ��Ӧ���ұ����ϵ�һ�ȴ���ֻ��2�֡�д��������������������H�Ľṹ��ʽ��__________��