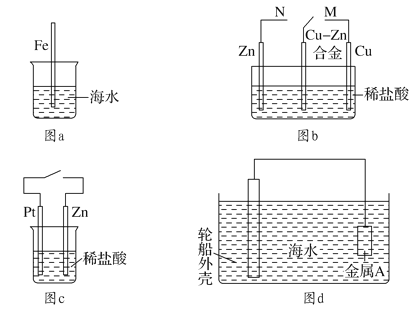
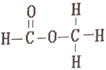ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΈΣ≤βΕ®Ρ≥”–ΜζΜ·ΚœΈοAΒΡΫαΙΙΘ§Ϋχ––»γœ¬ Β―ιΘΚ
Θ®1Θ©ΫΪ“ΜΕ®ΝΩΒΡ”–ΜζΈοA÷Ο”Ύ―θΤχ÷–≥δΖ÷»Φ…’Θ§ Β―ι≤βΒΟΘΚ…ζ≥…5.4g H2OΚΆ8.8g CO2Θ§œϊΚΡ―θΤχ6.72LΘ®±ξΉΦΉ¥Ωωœ¬Θ©Θ§‘ρΗΟ”–ΜζΈοΒΡ Β―ι Ϋ «__ΓΘ
Θ®2Θ©”Ο÷ ΤΉ“«≤βΕ®ΗΟ”–ΜζΜ·ΚœΈοΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΘ§ΒΟΒΫ»γΆΦ1Υυ ΨΒΡ÷ ΤΉΆΦΘ§‘ρΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ___Θ§ΗΟΈο÷ ΒΡΖ÷Ή” Ϋ «__ΓΘ
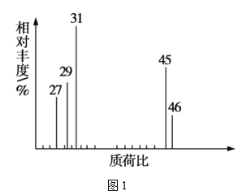
Θ®3Θ©ΗυΨίΦέΦϋάμ¬έΘ§‘Λ≤βAΒΡΩ…ΡήΫαΙΙ≤Δ–¥≥ωΫαΙΙΦρ ΫΘΚ___ΓΘ
Θ®4Θ©ΚΥ¥≈Ι≤’ώ«βΤΉΡήΕ‘”–ΜζΈοΖ÷Ή”÷–≤ΜΆ§ΈΜ÷ΟΒΡ«β‘≠Ή”Ηχ≥ω≤ΜΆ§ΒΡΈϋ ’ΖεΘ®–≈Κ≈Θ©Θ§ΗυΨίΈϋ ’ΖεΘ®–≈Κ≈Θ©Ω…“‘»ΖΕ®Ζ÷Ή”÷–«β‘≠Ή”ΒΡ÷÷άύΚΆ ΐΡΩΓΘάΐ»γ¬»ΦΉΜυΦΉΜυΟ―Θ®Cl-CH2-O-CH3Θ§”–2÷÷«β‘≠Ή”Θ©ΒΡΚΥ¥≈Ι≤’ώ«βΤΉ»γΆΦ2Υυ ΨΘΚ

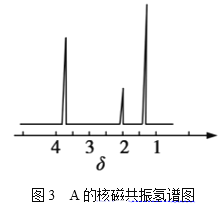
Ψ≠≤βΕ®Θ§”–ΜζΈοAΒΡΚΥ¥≈Ι≤’ώ«βΤΉΆΦ»γΆΦ3Υυ ΨΘ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ__ΓΘ
ΓΨ¥πΑΗΓΩC2H6O 46 C2H6O CH3CH2OHΓΔCH3-O-CH3 CH3CH2OH
ΓΨΫβΈωΓΩ
≈®ΝρΥα‘ω÷ΊΈΣΥ°ΒΡ÷ ΝΩΘ§Φν ·Μ“‘ω÷ΊΈΣΕΰ―θΜ·ΧΦΒΡ÷ ΝΩΘ§“‘¥ΥΩ…»ΖΕ®”–ΜζΈο÷–CΓΔH‘≠Ή”Ηω ΐ±»÷ΒΘ§ΫαΚœœϊΚΡ―θΤχΒΡΧεΜΐΩ…»ΖΕ®”–ΜζΈο÷–Ης‘≠Ή”Ηω ΐ±»÷ΒΘΜΗυΨί”–ΜζΈο‘≠Ή”Ηω ΐ±»÷ΒΩ…»ΖΕ®ΉνΦρ ΫΘ§ΫαΚœœύΕ‘Ζ÷Ή”÷ ΝΩΩ…»ΖΕ®”–ΜζΈοΖ÷Ή” ΫΘΜΗυΨί”–ΜζΈοΖ÷Ή” ΫΫαΚœΦέΦϋάμ¬έΩ…»ΖΕ®”–ΜζΈοΒΡΩ…ΡήΫαΙΙΘΜ‘ΌΗυΨί«βΤΉΆΦΩ…»ΖΕ®”–ΜζΈοΒΡΫαΙΙ ΫΘΜ“‘¥ΥΖ÷ΈωΓΘ
(1)…ζ≥…5.4g H2OΚΆ8.8g CO2Θ§H2OΒΡΈο÷ ΒΡΝΩΈΣ![]() Θ§Κ§0.6molΒΡ«β‘≠Ή”ΘΜCO2Έο÷ ΒΡΝΩΈΣ
Θ§Κ§0.6molΒΡ«β‘≠Ή”ΘΜCO2Έο÷ ΒΡΝΩΈΣ![]() Θ§Φ¥ΧΦΒΡΈο÷ ΒΡΝΩΈΣ0.2molΘ§O2ΒΡΈο÷ ΒΡΝΩΈΣ
Θ§Φ¥ΧΦΒΡΈο÷ ΒΡΝΩΈΣ0.2molΘ§O2ΒΡΈο÷ ΒΡΝΩΈΣ![]() Θ§‘ρ―θ‘≠Ή”ΒΡΈο÷ ΒΡΝΩΈΣ0.6molΘΜΗυΨίΖΫ≥Χ Ϋ÷–―θ‘≠Ή” ΊΚψΒΟΘ§”–ΜζΈο÷–ΒΡ―θ‘≠Ή”Έο÷ ΒΡΝΩΈΣ
Θ§‘ρ―θ‘≠Ή”ΒΡΈο÷ ΒΡΝΩΈΣ0.6molΘΜΗυΨίΖΫ≥Χ Ϋ÷–―θ‘≠Ή” ΊΚψΒΟΘ§”–ΜζΈο÷–ΒΡ―θ‘≠Ή”Έο÷ ΒΡΝΩΈΣ![]() Θ§‘ρ”–ΜζΈοCΓΔHΓΔOΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ2ΘΚ6ΘΚ1Θ§ Β―ι ΫΈΣC2H6OΘΜ
Θ§‘ρ”–ΜζΈοCΓΔHΓΔOΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ2ΘΚ6ΘΚ1Θ§ Β―ι ΫΈΣC2H6OΘΜ
(2)”…÷ ΤΉΆΦΩ…ΒΟœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ46Θ§‘ρΖ÷Ή” ΫΈΣC2H6OΘΜ
(3)”…AΖ÷Ή” ΫC2H6OΩ…÷ΣAΈΣ±ΞΚΆΜ·ΚœΈοΘ§ΆΤ≤βΤδΫαΙΙΦρ ΫΈΣCH3CH2OHΓΔCH3-O-CH3ΘΜ
(4)ΗυΨίAΒΡΚΥ¥≈Ι≤’ώ«βΤΉΆΦΩ…÷ΣA”–3÷÷≤ΜΆ§ΜΖΨ≥ΒΡH‘≠Ή”Θ§CH3-O-CH3÷Μ”–1÷÷«β‘≠Ή”Θ§CH3CH2OH”–3÷÷≤ΜΆ§ΜΖΨ≥ΒΡH‘≠Ή”Θ§Ι AΈΣCH3CH2OHΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ»ΐΗω»ίΜΐœύΆ§ΒΡΚψ»ίΟή±’»ίΤς÷–Α¥≤ΜΆ§ΖΫ ΫΆΕ»κΖ¥”ΠΈοΘ§ΖΔ…ζΖ¥”Π2SO2(g)ΘΪO2(g)![]() 2SO3(g)ΘΜΠΛH ΘΫΘ≠196.6kJ/molΘ§≤βΒΟΖ¥”ΠΒΡœύΙΊ ΐΨί»γœ¬ΘΚ
2SO3(g)ΘΜΠΛH ΘΫΘ≠196.6kJ/molΘ§≤βΒΟΖ¥”ΠΒΡœύΙΊ ΐΨί»γœ¬ΘΚ
»ίΤς1 | »ίΤς2 | »ίΤς3 | |
Ζ¥”ΠΈ¬Ε» T/K | 700 | 700 | 800 |
Ζ¥”ΠΈοΆΕ»κΝΩ | 2molSO2ΓΔ1mol O2 | 4molSO3 | 2molSO2ΓΔ1mol O2 |
ΤΫΚβv’ΐ(SO2)/ molΓΛLΘ≠1 ΓΛsΘ≠1 | v1 | v2 | v3 |
ΤΫΚβc(SO3)/ molΓΛLΘ≠1 | c1 | c2 | c3 |
Έο÷ ΒΡΤΫΚβΉΣΜ·¬ ΠΝ | ΠΝ1(SO2) | ΠΝ2(SO3) | ΠΝ3(O2) |
ΤΫΚβ≥Θ ΐK | K1 | K2 | K3 |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ « (ΓΓΓΓ)
A.v1ΘΦv2Θ§c2ΘΦ2c1B.K1ΘΨK3 Θ§p2ΘΨ2p3
C.v1ΘΦv