��Ŀ����
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32��+ I2 �� S4O62��+ 2I-
38.����100 mL0.0500 mol/L I2��Һ������Ҫ�������� ��ѡ���ţ���
a��100 mL����ƿ b����Ͳ c���ձ� d��������
�ζ��ܱ���ʹ���¶ȣ�20oC; �ζ��ܵ���С�̶�Ϊ mL��
39.ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500 mol/L I2��Һ�ζ���ʵ����������(��3�γ�����Ϊ 0.00���յ������ͼ; ���ʲ��μӷ�Ӧ)��
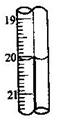
����ζ��յ�������� ��
Na2S2O3?5H2O(ʽ��248)�����������ǣ�����4λС���� ��
40.�����ʵ����ƫ�͵IJ����� ��ѡ���ţ���
a. �ζ�ʱ�ζ����е�Һ�������ƿ��
b. ��ƿ������ˮϴ��������װ�������Һ
c. δ�ñ�Һ��ϴ�ζ���
d. ��ʱ��Һ������ƿ��
38.����100 mL0.0500 mol/L I2��Һ������Ҫ�������� ��ѡ���ţ���
a��100 mL����ƿ b����Ͳ c���ձ� d��������
�ζ��ܱ���ʹ���¶ȣ�20oC; �ζ��ܵ���С�̶�Ϊ mL��
39.ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500 mol/L I2��Һ�ζ���ʵ����������(��3�γ�����Ϊ 0.00���յ������ͼ; ���ʲ��μӷ�Ӧ)��

| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 | |
Na2S2O3?5H2O(ʽ��248)�����������ǣ�����4λС���� ��
40.�����ʵ����ƫ�͵IJ����� ��ѡ���ţ���
a. �ζ�ʱ�ζ����е�Һ�������ƿ��
b. ��ƿ������ˮϴ��������װ�������Һ
c. δ�ñ�Һ��ϴ�ζ���
d. ��ʱ��Һ������ƿ��
38.b��1�֣���0.1��1�֣�.
39.��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��1�֣�����ȫ�����֣���0.9920��99.20%��1�֣�.
40.d��1�֣�
39.��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��1�֣�����ȫ�����֣���0.9920��99.20%��1�֣�.
40.d��1�֣�
���������38.����100 mL0.0500 mol/L I2��Һ��I2��Ҫ����������Ҫ����������Ͳ���ζ��ܵ���С�̶�Ϊ0.1mL��
39.ָʾ��Ϊ���ۣ���I2�������ʵ���ζ��յ����������Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��
ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���ƷΪ0.500g����3�ζ���Ϊ20.10mL����ֵ��ǰ�������ϴ���ȥ��
��2S2O32��+ I2 �� S4O62��+ 2I-
n(Na2S2O3��5H2O)=2n(I2)=2��0.0500 mol/L��
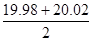 ��10-3L=0.002mol
��10-3L=0.002mol�ʦ�(Na2S2O3��5H2O)=
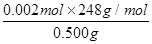 ��100%=99.2%��
��100%=99.2%��40.a���ζ�ʱ�ζ����е�Һ�������ƿ�⣬�ζ�����ƫ��ʵ�����ߣ�b����ƿ������ˮϴ��������װ�������Һ��Na2S2O3�ļ������䣬ʵ������c��δ�ñ�Һ��ϴ�ζ��ܣ���ζ����ڱڸ���ˮ������ı�Һ��ϡ�ͣ��ʵζ���Ҫ�����ƫ��ʵ�����ߣ�d����ʱ��Һ������ƿ�⣬Na2S2O3����ʧ���ζ���Ҫ�ı�Һ���ƫС��ʵ�����͡�

��ϰ��ϵ�д�
�����Ŀ


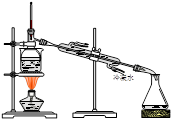
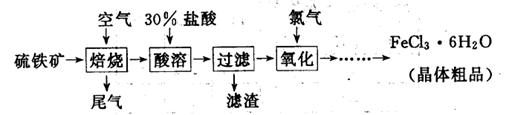
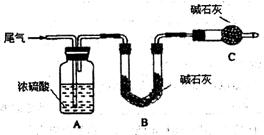
 6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .



