��Ŀ����
����˵������ȷ����
| A����Ħ����ʱ�þƾ�ϴ�Ӳ�Ʒ���ư�˾ƥ��ʱ����ˮϴ�Ӳ�Ʒ |
| B��������������ʱ��Ϊ�õ��ϴ�����ľ��壬Ӧ��Ȼ��ȴ�������ù�ҹ |
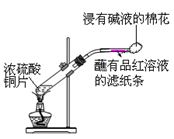
| C��������пǰ����Ҫ��ɰֽ��ĥֱ�������ù⻬��Ȼ���������������Һ�н���5���ӣ���ˮϴ���������ϡ�����н��ݣ�����ˮϴ�� |
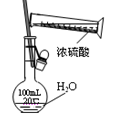
| D������ʳ�����Ậ���IJⶨ��ʵ��ʱ�������Ƚ�����ʳ��ϡ��10����Ȼ����ϴ������ʽ�ζ�����ȡһ�������ϡ��Һ�ڽྻ����ƿ�У���ƿ����Ҫ���ô�����Һ��ϴ |
D
���������A��Ħ�������л��ܼ��е��ܽ��С�������Ħ����ʱ�þƾ�ϴ�Ӳ�Ʒ�����Լ��پ�����ܽ⣻��˾ƥ�ֵ��ܽ�����¶ȵ����߶���������ư�˾ƥ��ʱ����ˮϴ�Ӳ�Ʒ���Լ��ٹ�����ܽ⣬A��ȷ��B�������¶Ƚϵ�ʱ�����Ͷ�Ҳ�ϵͣ�������������С�����ϣ�����������������ʱ��Ϊ�õ��ϴ�����ľ��壬Ӧ��Ȼ��ȴ�������ù�ҹ��B��ȷ��C��������пǰ����Ҫ��ɰֽ��ĥֱ�������ù⻬��Ȼ���������������Һ�н���5���ӣ�Ŀ���dz�ȥ��������ۡ���ˮϴ���������ϡ�����н��ݣ�Ŀ���dz�ȥ��������⣬����ˮϴ�����ɣ�C��ȷ��D����ȡ�������ʽ�ζ��ܱ��������ñ�Һ��ϴ��D����ȷ����ѡD��

��ϰ��ϵ�д�
�����Ŀ

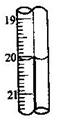




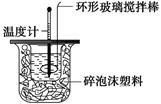
 �¶�
�¶� ��
�� ���ǣ� ��
���ǣ� ��