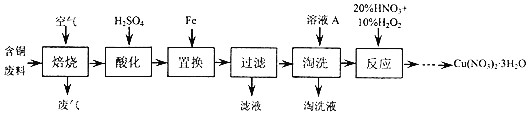
��Ŀ����
1��ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
������������̣��ش��������⣺
��1�����������õ��IJ����������ձ����������⣬��������250mL����ƿ����ͷ�ιܣ�
�����������ƣ�
��2����д��������ˮ���������ӷ�Ӧ����ʽ2Fe2++Br2=2Fe3++2Br-��
��3������������ȣ���ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1-b2=0.3g�����������Ӧ���еIJ������ٴμ�����ȴ��������ֱ������������С��0.1g���������������� W1 g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������$\frac{1120��W{\;}_{2}-W{\;}_{1}��}{160a}$��100%����ͬѧ����������Բ������·������ⶨ��
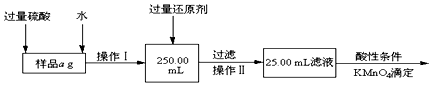
���ܽ���Ʒ���������ᣬ���������ᣬԭ���ǹ���������Ժ���KMnO4�ĵζ��и��ţ�
��ѡ��Ļ�ԭ���Ƿ��������� ����ǡ�����ԭ���ǣ������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ��
�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������$\frac{2.8bc}{a}$��
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
��2������Br2���������ԣ�������Fe2+��Ϊ��ʹFe3+��ֳ�������ˮҪ������
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
���ܽ���Ʒ�������ᣬ�ø���������õζ�ʱ�����������ӣ�Ӱ��ʵ��ⶨ�����
��ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص���������������������ԭ�����������ᷴӦ�����������ӣ���������Ԫ�ص����Բⶨ���������
�����ݸ�����غ��������ӵ�������ԭ��Ӧ������ϵ���㣮
��� �⣺��1��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��2����Br2���������ԣ�������Fe2+��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�������ˮҪ�������ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��
��3��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��$\frac{112}{160}$����Ʒ����Ԫ�ص�����������$\frac{1120��W{\;}_{2}-W{\;}_{1}��}{160a}$��100%��
�ʴ�Ϊ���ٴμ�����ȴ��������ֱ������������С��0.1g��$\frac{1120��W{\;}_{2}-W{\;}_{1}��}{160a}$��100%��
�ٸ�����ؾ���ǿ�����ԣ��������ᣬ��Һ�е������ӻᱻ�����������ĸ�����أ�����ʵ��ⶨ���ʴ�Ϊ������������Ժ���KMnO4�ĵζ��и��ţ�
�ڻ�ԭ��������������Ϊ�����ֻ�������ᷴӦ�����������������ø�����صζ�������������������������ԭ��������Ԫ�صIJⶨ��
�ʴ�Ϊ���������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ��
�����ݷ�Ӧ5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O�����ݶ�����ϵ����õ�������Ԫ����������ΪX%��
5Fe2+��5Fe3+��KMnO4
5��56 1
a��X%��$\frac{25.00}{250.0}$ c��b��10-3
��Ԫ�ص�����������X%=$\frac{2.8bc}{a}$���ʴ�Ϊ��$\frac{2.8bc}{a}$��
���� ������Ҫ��������Ԫ�ص����������IJⶨ��ʵ�������ʵ�����ݵļ���Ӧ�ã�ͬʱ������ʵ��֪ʶ�ķ����жϣ��ѶȲ���

| A | B |
| ��ʹ������Ȼ�̼��Һ��ɫ�� �ڱ���ģ��Ϊ�� 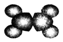 ������ˮ��һ�������·�Ӧ���ɴ� | ����C��H����Ԫ����ɣ� �����ģ��Ϊ��  |
��1��A�������ǣ���ϩ
��2��д����һ�������£�A���ɸ߷��ӻ�����Ļ�ѧ��Ӧ����ʽ
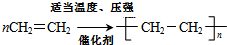 ��
����3��B������Ϊ������B���ұ���Ϊͬϵ������ұ���ͬ���칹�廹3 �֣�д������һ�ֵ����������ڶ��ױ��������ױ���Զ��ױ�����
| A�� | ij��Һ$\stackrel{+ʯ��}{��}$��Һ�ʺ�ɫ��ԭ��Һ������Һ | |
| B�� | ij��Һ$\stackrel{+�����ữ}{��}$����������$\stackrel{+BaCl_{2}��Һ}{��}$�а�ɫ������ԭ��Һ�к�SO42- | |
| C�� | ij��Һ$\stackrel{+��̪}{��}$��Һ�ʺ�ɫ��ԭ��Һ�Ǽ���Һ | |
| D�� | ij��Һ$\stackrel{+ϡ����}{��}$������ɫ��ζ���壬˵��ԭ��Һ����CO32- |
| A�� | �þƾ���ȡ��ˮ�е��嵥�ʵIJ�����ѡ�÷�Һ©���������÷�Һ | |
| B�� | ��Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| C�� | ������ƽ���������ϸ���һ����ͬ������ֽ���ٰ��������ƹ������ֽ�ϳ� | |
| D�� | ��ȡ����Һǰ����Է�Һ©����© |
| A�� | ���� | B�� | �� | C�� | �Ȼ�����Һ | D�� | CuSO4��Һ |
| A�� | ij��Һ��ˮ�������c��H+��=10-13�������Һ��pHһ��Ϊ13 | |
| B�� | pH=4.5�ķ���֭��c��H+����pH=6.5��ţ����c��H+����2�� | |
| C�� | pH��ͬ�İ�ˮ������������ϣ�������ҺpH��7 | |
| D�� | pH=7��CH3COOH��CH3COONa�����Һ�У�c��Na+��=c��CH3COO-�� |
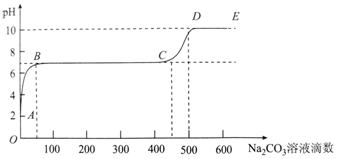
| A�� | ��ʼʱ��ҺpH=2����Ϊ����Һ�л�������ʣ�� | |
| B�� | BC�α�ʾ����̼������Һ�ĵ��룬CaCO3���������������� | |
| C�� | AB�����ķ�ӦΪ��Ca2++CO32-�TCaCO3�� | |
| D�� | ����500��̼������Һ����Һ��c��OH-����c��H+�� |