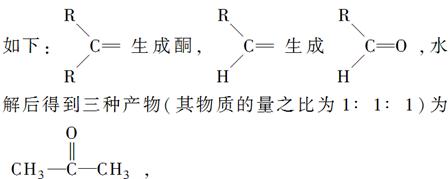
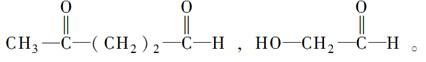��Ŀ����
��ȩ��ʳƷ��ҽҩ�������ȷ��涼��Ӧ�á����������dz����ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
��1�����ȩ��C��H��O����Ԫ����ɣ����������ȩ���ӵ���Է�������Ϊ132���������̼Ԫ�ص���������Ϊ81��8%�����ȩ�ķ���ʽ�� ��
���ȩ�DZ���һȡ����˴Ź���������ʾ�����������������ֲ�ͬ��ѧ��������ԭ�ӣ���ṹ��ʽ�� ����������˳���칹���ӳ�칹��
��2����֪��
I��ȩ��ȩ�ܷ�����Ӧ��ԭ�����£�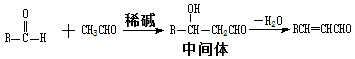
II���ϳ����ȩ�Ĺ�ҵ��������ͼ��ʾ�����м�Ϊij������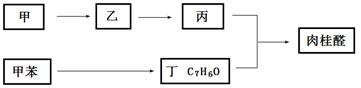
�ټĽṹ��ʽ�� ��
�ڱ��Ͷ��������Ļ�ѧ����ʽ�� ��
��3�����ȩ�ܱ�������Һ�������پ��ữ�õ�����ᣬд�����������Ľṹ��ʽ ��
��4�����÷�����AΪԭ�Ϻϳ���������H��·�����£����A�ĺ˴Ź���������ͼ��6���壬�����֮��Ϊ1�U2�U2�U2�U1�U2����
�ٻ�����F�еĹ������� �������ƣ���
��B��C�ķ�Ӧ������ ��F��G�ķ�Ӧ������ ��
����д��ѧ����ʽ
F��I
G��H
��G��ͬ���칹���У���������Ŀ������ֻ��һ��ȡ������ͬ���칹���� �֡�
��ṹ��ʽ�ֱ���
��1��C9H8O ��1�֣� 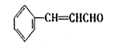 ��2�֣�
��2�֣�
��2����CH2=CH2��2�֣�
��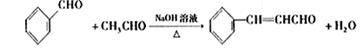 ��2�֣�
��2�֣�
��3��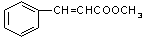 ��2�֣�
��2�֣�
��4�����ǻ����Ȼ� ��2�֣�
��ˮ�ⷴӦ����ȡ����Ӧ������ȥ��Ӧ ����1�֣�
��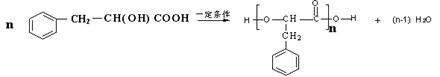 ��2�֣�
��2�֣� ��2�֣�
��2�֣�
�� 4 ��1�֣�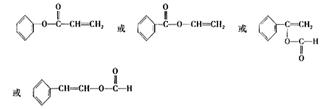 ��2�֣�
��2�֣�
���������������1�����ȩ����Է�������Ϊ132���������̼Ԫ�ص���������Ϊ81��8%�������ȩ�ķ����к�9��̼ԭ�ӣ�ȩ������Է�������Ϊ29����ʣ�µ�����ӦΪ103������������8��̼ԭ�ӣ�������������ԭ����7���������ȩ�ķ���ʽΪC9H8O�����ȩ�DZ���һ����҂�������3����ԭ�ӣ����к�ȩ�������Խṹ��ʽΪ
��2������ȩ��ȩ�ķ�Ӧԭ����֪�����ȩӦ�ɱ���ȩ����ȩ��Ӧ�õ������Ϊ��ȩ��������������������ȩ�����Լ�Ӧ����ϩ��ˮ�����Ҵ�������������ȩ����ϩ�Ľṹ��ʽΪCH2=CH2
����ȩ�뱽��ȩ��Ӧ�Ļ�ѧ����ʽ������Ŀ�е���֪���ɵó���Ϊ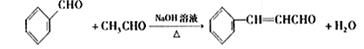
��3��������Һֻ����ȩ�����������������Ľṹ��ʽ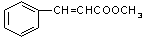
��4����A�ĺ˴Ź���������ͼ��6���壬�����֮��Ϊ1�U2�U2�U2�U1�U2���ж�A�е�̼̼˫���ڂ����Ķ�λ���ɴ��ж�A��B�����ӳɷ�Ӧ��B��C����ˮ�ⷴӦ��C��D����������Ӧ��D��E����������Ӧ��E��F�����ʻ����ⷴӦ������D�к��Ȼ����ǻ���
���ɷ�Ӧ�����ж�B��C�ķ�Ӧ������ˮ�ⷴӦ��F��G�ķ�Ӧ��������ȥ��Ӧ��
��F��I�������۷�Ӧ����ѧ����ʽΪ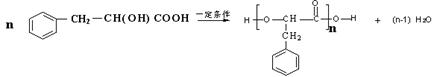
G��H����������Ӧ����ѧ����ʽΪ
��GΪ����ᣬ������������ұ���ֻ��һ��ȡ������ͬ���칹���������4�֣��ֱ���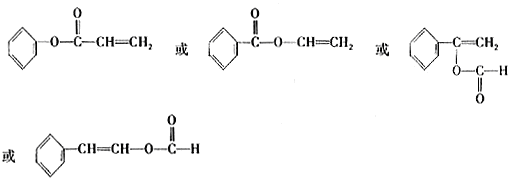
���㣺�����л������ʽ��ȷ�����ṹ��ʽ����ѧ����ʽ����д����Ӧ���͵��жϣ�ͬ���칹����ж�

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д���ˮ�����Ǻϳ����������Ȼ�������Ҫ���裬ij�����ϳ�·�����£�
��1���������Ļ�ѧʽΪ ��
��2��������A�Ľṹ��ʽΪ ����3����Ӧ�ٵĻ�ѧ����ʽΪ ��
��4������˵��������� ��
| A����������ܷ���������Ӧ | B��������������ڷ����� |
| C����Ӧ������������Ӧ | D�������������4 molH2�����ӳɷ�Ӧ |
��5����������뻯�����Ϊͬ���칹�壬���к���������������FeCl3��Һ������ɫ��Ӧ���䱽���ϵ�һ�ȴ���ֻ��2�֡�д��һ���������������Ģ��Ľṹ��ʽ�� ��
��6�����������ͼ��ʾ����һ��������Ҳ�ܷ������������ڢܲ��Ļ�����Ӧ���������������Ӧ����Ľṹ��ʽΪ ��


 ��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��
��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��