��Ŀ����
4������������SnSO4�������ڶ�����ҵ��ijС����Ƶ�SnSO4�Ʊ�·����ͼ��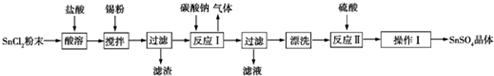
��֪��
�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ�������
��SnCl2��ˮ�����ɼ�ʽ�Ȼ�������
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ��ͬ����A�壬��λ�����ڱ��ĵ������ڣ�
��2���õ���SnSO4�����Ǻ������ᾧˮ�ľ��壬��֪������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵȣ�
��3���ܽ�SnCl2��ĩ���Ũ���ᣬԭ����SnCl2ˮ�ⷴӦΪSnCl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣮
��4���������۵��������������ٵ�����ҺpH���ڷ�ֹSn2+��������
��5����Ӧ��õ��ij�����SnO���õ��ó��������ӷ���ʽ��Sn2++CO32-�TSnO��+CO2����
��6�����������£�SnSO4��˫��ˮ��Ӧ�����ӷ���ʽ��Sn2++H2O2+2H+�TSn4++2H2O��
��7����С��ͨ�����з����ⶨ�������۵Ĵ��ȣ����ʲ����뷴Ӧ����
�ٽ��������ڹ����������У������ķ�ӦΪSn+2HCl�TSnCl2+H2����
�ڼ��������FeCl3��Һ��
������֪Ũ�ȵ�K2Cr2O7��Һ�ζ��������ɵ�Fe2+���ټ������۵Ĵ��ȣ�
����ƽ����ʽ��
6FeCl2+1K2Cr2O7+14HCl�T6FeCl3+2KCl+2CrCl3+7H2O��
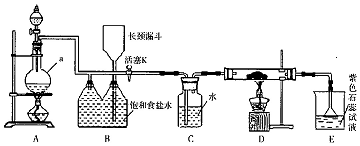
���� Ϊ��ֹSnCl2ˮ�⣬���Խ�SnCl2�ܽ���ϡ�����У�Ȼ��������۽��裬��ֹSn2+��������Ȼ�������ȥ������SnCl2�ܽ�õ���Һ����̼���Ƴ��������ӣ�����Һ�м���̼���ƣ�������ӦSn2++CO32-�TSnO��+CO2����Ȼ����˵õ�������������ϴ�ӣ��������ܽ�õ�������������������Һ����Ũ������ȴ�ᾧ������õ����������壬
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ������ͬһ���壬���ڢ�A�壬����ԭ������������������Ԫ������ȷ�����ڵ����ڣ�
��2��������ͼ��֪���������Ǵ���Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ���
��3������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ��������������ᣬ����Sn2+ˮ�⣻
��4������Ϣ��֪��Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��5����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�Ϊ�仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼��
��6�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ��
��7�����ݵ���ת���غ���ƽ��д��Ӧ�Ļ�ѧ����ʽ��
��� �⣺��1����Ԫ����̼Ԫ������ͬһ���壬���ڢ�A�壬ԭ�Ӻ˵����Ϊ50����50-2-8-8-18=14����Sn���ڵ������ڣ��������ڱ��е�λ��Ϊ���������ڵڢ�A�壬
�ʴ�Ϊ���壻
��2��������ͼ��֪���������Ǵ���Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ���
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��3������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��Sn Cl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣬
�ʴ�Ϊ��SnCl2ˮ�ⷴӦΪSnCl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
��4������Ϣ��֪��Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
�ʴ�Ϊ����ֹSn2+��������
��5����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�Ϊ�仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼�����ӷ���ʽΪ��Sn2++CO32-�TSnO��+CO2����
�ʴ�Ϊ��Sn2++CO32-�TSnO��+CO2����
��6�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
�ʴ�Ϊ��Sn2++H2O2+2H+�TSn4++2H2O��
��7����Ԫ�ػ��ϼ�+2�����ߵ�+3�ۣ�CrԪ�ػ��ϼ�+6�۱仯Ϊ+3�ۣ�����ת������6e-�����ԭ���غ�͵����غ������ȱ��Ϊˮ����ƽ��д��ѧ����ʽΪ��6FeCl2+K2Cr2O7+14HCl�T6FeCl3+2KCl+2CrCl3+7H2O
�ʴ�Ϊ��6��1��14��6��2��2��7��H2O��
���� ������SnSO4�Ʊ�Ϊ���壬����ѧ���Թ������̵����⡢���ʵķ����ᴿ���Ķ���Ŀ��ȡ��Ϣ�����������û�ѧ������д���ζ�Ӧ�ü����ù�ϵʽ���еļ���ȣ��Ѷ��еȣ���ѧ���Ļ���֪ʶ���������нϸߵ�Ҫ��

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�| A�� | 0.308 | B�� | 308 | C�� | 154 | D�� | 0.154 |
��CS2+3Cl2$\frac{\underline{\;95-100��\;}}{\;}$CCl4+S2Cl2��
��2S+Cl2$\frac{\underline{\;111-140��\;}}{\;}$S2Cl2��
��֪��S2Cl2����ˮ��Ӧ��S2Cl2+Cl2$\stackrel{��}{?}$2SCl2��

�±��Ǽ������ʵ��۷е㣬��ͼ�Ƿ�Ӧװ��ͼ��
| ���� | �е�/�� | �۵�/�� |
| S | 445 | 113 |
| CS2 | 47 | -109 |
| CCl4 | 77 | -23 |
| S2Cl2 | 137 | -77 |
��2����װ��C�����ɸ���ܣ���װ��C�п�ѡ�õĹ����Լ�����ˮCaCl2�������P2O5����
��3��Dװ���������ܵ���������������������������Ӧ������Dװ����ƿ�ڵĻ�����з��������ķ���������
��4��S2Cl2������ˮ��Ӧ�л�ɫ�������ɣ���������ɫ������ʹƷ����Һ��ɫ����÷�Ӧ�Ļ�ѧ����ʽΪ2S2Cl2+2H2O�T3S��+SO2��+4HCl����
��5��Ϊ������ƵõIJ�ƷS2Cl2�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶ȺͿ���Ũ����ĵ��ٲ���̫�죮
��6����ͼβ��װ�ò������ƣ��Ľ���ʩ����D��E֮�����Ӹ���װ�ã�ͬʱβ������Ҫ��������
��7����A��������26.1g MnO2���õ�10.8g��Ʒ����ʵ��IJ�����80%��
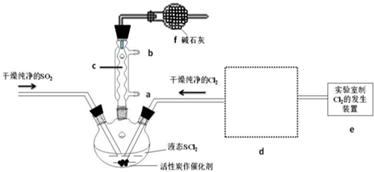
��1������c�����������������ܣ�װ��f�������������ݳ��ж���Cl2��SO2����ֹ�����е�ˮ�������뷴Ӧװ�ã���ֹSOCl2ˮ�⣮
��2��SOCl2��ˮ��Ӧ�Ļ�ѧ����ʽΪSOCl2+H2O=SO2��+2HCl��������AlCl3��Һ���ܵõ���ˮAlCl3��ʹSOCl2��AlCl3•6H2O��ϼ��ȣ��ɵõ���ˮAlCl3���Խ���ԭ��AlCl3��Һ��ˮ�⣬AlCl3•6H2O��SOCl2��ϼ��ȣ�SOCl2��AlCl3•6H2O�еĽᾧˮ���ã�������ˮAlCl3��SO2��HCl���壬SOCl2��ˮ������SO2��HCl����AlCl3ˮ�⣮
��3�����������Ʊ�SO2�ķ��������ѡ���Ƕ���
| ���� | �� | �� | �� | �� |
| ����װ�� |  |  |  |  |
| ��ѡ�Լ� | NaHSO3���� | 18.4mol/LH2SO4+Cu | 4mol/LHNO3+Na2SO3 | 70%H2SO4+K2SO3 |
��5�����������������ƿ�л������뿪��ʵ�������������֪SCl2�ķе�Ϊ50�棩����Ӧ�����ĵ�Cl2�����Ϊ896ml����ת��Ϊ��״����SO2�����������õ�������SOCl24.76g����SOCl2�IJ���Ϊ50% ��������λ��Ч���֣���
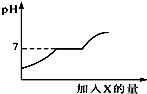 ���������CaCl2�Ļ����Һ����������μ������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�ǣ�������
���������CaCl2�Ļ����Һ����������μ������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�ǣ�������| A�� | ˮ | B�� | ����ʯ��ˮ | C�� | ������Һ | D�� | ϡ���� |