��Ŀ����
��14�֣���֪�������£�A�����ҺpH��a��B�����ҺpH��b��
��1����AΪ���ᣬBΪ�������ƣ���a��b��14�����ߵ������ϣ�����Һ��pH�� �����������Ϊ1��10��Ϻ���Һ�����ԣ���a��b�� ��
��2����AΪ���ᣬBΪ������������a��4��b��12����ôA��Һ��ˮ�������������Ũ��Ϊ mol��L��1��B��Һ��ˮ�������������Ũ��Ϊ ��mol��L��1
��3����AΪ���ᣬBΪ�������ƣ���a��b��14�������ΪVA�Ĵ�������ΪVB������������Һ��Ϻ���Һ�����ԣ����������ϵVA VB����Ϻ���Һ�е�����Ũ�ȹ�ϵΪc(Na��) c(CH3COO��) ���������������������
��4����A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a��b��14�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽΪ ��
��1����AΪ���ᣬBΪ�������ƣ���a��b��14�����ߵ������ϣ�����Һ��pH�� �����������Ϊ1��10��Ϻ���Һ�����ԣ���a��b�� ��
��2����AΪ���ᣬBΪ������������a��4��b��12����ôA��Һ��ˮ�������������Ũ��Ϊ mol��L��1��B��Һ��ˮ�������������Ũ��Ϊ ��mol��L��1
��3����AΪ���ᣬBΪ�������ƣ���a��b��14�������ΪVA�Ĵ�������ΪVB������������Һ��Ϻ���Һ�����ԣ����������ϵVA VB����Ϻ���Һ�е�����Ũ�ȹ�ϵΪc(Na��) c(CH3COO��) ���������������������
��4����A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a��b��14�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽΪ ��
��1��7 13 ��2��10�D10 10�D12 ��3���� ��
���⿼�����pHֵ���㼰�������Һ��Ҫ֪�����������ǿ�����֮������𡪴��ڵ���ƽ�⡣
��1�� ���Խ�����ѧ���������������������һС�⡣�����Ũ��Ϊ0.1mol/L,���Ũ��Ϊ0.1 mol/L��a��b��14�������ߵ������ϣ���Һ��Ȼ�����ԣ�pH=7����Ϻ���Һ���ԣ�H+���ʵ�������OH-�����ʵ������������Ϊ1��10������Ũ��֮��Ϊ10��1�������Ũ��Ϊ0.1mol/L,����Ũ��Ϊ0.01 mol/L������a��b��13.
��2�� ��֪����ˮ�������������Ũ��=����������Ũ�Ⱥ�C(H+)��C(OH-)=10-14�����ˮ���������C(H+)=C(OH-)=10-14/10-4=10�D10�����ڼˮ���������C(H+)= 10�D12 ��ȫ��������ˮ�ĵ��룩��
��3�� ���ǿ�����ô�룺����������ϣ���ҺӦ�������ԣ���Ϊ������������ʣ���ϵ���������ӣ�������VA < VB�����ݵ���غ㣬����C(H+)=C(OH-)������c(Na��) = c(CH3COO��)
��4�� ���������жϣ���ǿ���������M+ + H20 ="=" MOH + H+
��1�� ���Խ�����ѧ���������������������һС�⡣�����Ũ��Ϊ0.1mol/L,���Ũ��Ϊ0.1 mol/L��a��b��14�������ߵ������ϣ���Һ��Ȼ�����ԣ�pH=7����Ϻ���Һ���ԣ�H+���ʵ�������OH-�����ʵ������������Ϊ1��10������Ũ��֮��Ϊ10��1�������Ũ��Ϊ0.1mol/L,����Ũ��Ϊ0.01 mol/L������a��b��13.
��2�� ��֪����ˮ�������������Ũ��=����������Ũ�Ⱥ�C(H+)��C(OH-)=10-14�����ˮ���������C(H+)=C(OH-)=10-14/10-4=10�D10�����ڼˮ���������C(H+)= 10�D12 ��ȫ��������ˮ�ĵ��룩��
��3�� ���ǿ�����ô�룺����������ϣ���ҺӦ�������ԣ���Ϊ������������ʣ���ϵ���������ӣ�������VA < VB�����ݵ���غ㣬����C(H+)=C(OH-)������c(Na��) = c(CH3COO��)
��4�� ���������жϣ���ǿ���������M+ + H20 ="=" MOH + H+

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ

 ��������ͼ�л����ζ���������Һ��pH�����μ�����������Һ����ı仯������ͼ��Ҫ���A�㣩��
��������ͼ�л����ζ���������Һ��pH�����μ�����������Һ����ı仯������ͼ��Ҫ���A�㣩��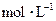 B��Һ������Ũ���ɴ�С���� ��
B��Һ������Ũ���ɴ�С���� ��