��Ŀ����
��Ļ������������Ϳ����з�������Ҫ���á�
��1��SO2Cl2����������ҽҩƷ��Ⱦ�ϡ�������Լ��ȡ���֪��SO2Cl2(g) SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______��
SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______��
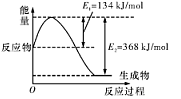
��2����ҵ���Ʊ�����Ĺ����д��ڷ�Ӧ��2SO2(g)��O2(g) 2SO3(g) ��H����198kJ��mol��1400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��
2SO3(g) ��H����198kJ��mol��1400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��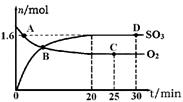
��0��20min��Ӧ��ƽ�����ʦ�(O2)��___________��
������������ȷ���� ��
a��A�����(SO2)������(SO2)
b��B�㴦��ƽ��״̬
c��C���D��n(SO2)��ͬ
d�������������䣬500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵ��
��3����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L��1��Na2SO3��Һ������ҺpHԼΪ6ʱ��Na2SO3��Һ����SO2�����������½���Ӧ�������ռ�����ʱ��Һ��c (SO32��)��Ũ����0.2 mol��L��1������Һ��c(HSO3��)��_________mol��L��1��
��17�֣���1��17.5��3�֣� 1.62��3�֣� 46.6g��3�֣�
��2��0.02mol(L��min) ��3�֣� ac��2�֣� ��3��1.6mol/L��3�֣�
���������������1���ﵽƽ��ʱ�������к�0.18mol SO2��������Ȼ�ѧ����ʽ��֪���˹��̷�Ӧ���յ�����Ϊ97.3 kJ��mol��1��0.18mol��17.5kJ�����ݷ���ʽ��֪
SO2Cl2(g) SO2(g)��Cl2(g)
SO2(g)��Cl2(g)
��ʼŨ�ȣ�mol/L�� 0.20 0 0
ת��Ũ�ȣ�mol/L�� 0.18 0.18 0.18
ƽ��Ũ�ȣ�mol/L�� 0.02 0.18 0.18
���Ը��¶��·�Ӧ��ƽ�ⳣ��K��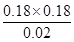 ��1.62��
��1.62��
���û��������������BaCl2��Һ�У�������ӦSO2��Cl2��2H2O��H2SO4��2HCl�����Դ�ʹƽ�����������Ӧ������У�����������ɳ��������ʵ�����0.20mol�������ᱵ������Ϊ0.20mol��232g/mol��46.6g��
��2���ٸ���ͼ���֪20minʱ������������ʵ�����1.6mol�����������������ʵ�����0.8mol��Ũ����0.4mol/L������0��20min��Ӧ��ƽ�����ʦ�(O2)��0.4mol/L��20min��0.02mol(L��min)��
��a������ͼ���֪A�㷴Ӧû�дﵽƽ��״̬��ƽ��������Ӧ������У������(SO2)������(SO2)��a��ȷ��b��B�����ʵ�Ũ����Ȼ�DZ仯�ģ���Ӧû�д���ƽ��״̬��b����ȷ��c��C���D�������ͬ�����µ�ƽ��״̬�����n(SO2)��ͬ��c��ȷ��d������Ӧ�Ƿ��ȷ�Ӧ�������������䣬�����¶�ƽ�����淴Ӧ�����ƶ�������500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵС��d����ȷ����ѡac��
��3����Һ��c (SO32��)��Ũ����0.2 mol��L��1��������c (SO32��)��Ũ�ȣ�1.0mol/L��0.2mol/L��0.8mol/L����˸��ݷ���ʽSO32����SO2��H2O��2HSO3����֪��Һc(HSO3��)��0.8mol/L��2��1.6mol/L��
���㣺���鷴Ӧ�ȡ���Ӧ���ʡ�ƽ�ⳣ�����йؼ��㣻���������ƽ��״̬��Ӱ���Լ���Һ������Ũ�Ⱥͳ������ɵ��йؼ���

 ��У����ϵ�д�
��У����ϵ�д��״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
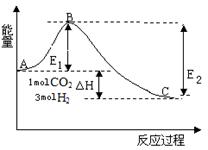
(1)��ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO(g)��2H2(g) CH3OH(g)����H1
CH3OH(g)����H1
��Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)����H2
CH3OH(g)��H2O(g)����H2
��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ�����________(���)��
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)��
| �¶� | 250 �� | 300 �� | 350 �� |
| K | 2.041 | 0.270 | 0.012 |
�ɱ��������жϣ���H1______0(�����������������)��
��ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)��0.2 mol��L��1����CO��ת����Ϊ________����ʱ���¶�Ϊ________(���ϱ���ѡ��)��
(2)��֪�ڳ��³�ѹ�£�
��2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(g) ��H1����1 275.6 kJ��mol��1
��2CO(g)��O2(g)=2CO2(g) ��H2����566.0 kJ��mol��1
��H2O(g)=H2O(l)����H3����44.0 kJ��mol��1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
__________________________________________________________��
����̼ѭ��������������ҵĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2����Ϊ��ѧ���о�����Ҫ���⡣
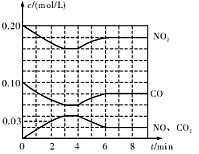
��1������ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ
CO(g)��H2O(g) CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1��6 | 2��4 | 6 |
| 2 | 900 | 2 | 1 | 0��4 | 1��6 | 3 |
| 3 | 900 | a | b | c | d | t |
��ʵ��2������ƽ�ⳣ��K= ��
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b ��ֵ_______(�����ֵ��ȡֵ��Χ)��
��ʵ��4����900��ʱ���ڴ������м���CO��H2O��CO2��H2��Ϊ1mol�����ʱV�� V�����<�� ����>�� ����=������
��2����֪�ڳ��³�ѹ�£�
��2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H����1275.6 kJ��mol
��2CO (g)+ O2(g) �� 2CO2(g) ��H����566.0 kJ��mol
��H2O(g) �� H2O(l) ��H����44.0 kJ��mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��____________
��3����֪������һ�ֶ�Ԫ���ᣬ�������ƣ�NaHC2O4����Һ�����ԡ������£���10 mL 0.01 mol��L-1 H2C2O4��Һ�еμ�10mL 0.01mol��L-1 NaOH��Һʱ���Ƚ���Һ�и�������Ũ�ȵĴ�С��ϵ ��
��4��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8��10-9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2��10-4mol/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ _______________mol/L��
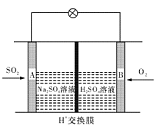
��5���Զ����ѣ�CH3OCH3����������H2SO4Ϊԭ�ϣ���Ϊ�缫�ɹ���ȼ�ϵ�أ��乤��ԭ�������ȼ�ϵ�ص�ԭ�����ơ���д���õ�ظ����ϵĵ缫��Ӧʽ�� ��
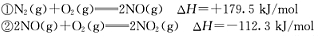
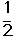 O2(g) ��
O2(g) ��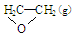 ; ��H1 ��C2H4(g)+3O2(g) �� 2CO2(g)+2H2O(g); ��H2
; ��H1 ��C2H4(g)+3O2(g) �� 2CO2(g)+2H2O(g); ��H2 CH3OH(g) +H2O(g) ��H =��49.0 kJ��mol��1
CH3OH(g) +H2O(g) ��H =��49.0 kJ��mol��1